Abstract
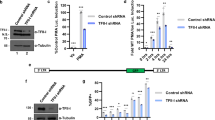
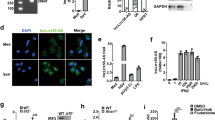
The molecular mechanism by which roquin controls the expression of inducible costimulator (ICOS) to prevent autoimmunity remains unsolved. Here we show that in helper T cells, roquin localized to processing (P) bodies and downregulated ICOS expression. The repression was dependent on the RNA helicase Rck, and roquin interacted with Rck and the enhancer of decapping Edc4, which act together in mRNA decapping. Sequences in roquin that confer P-body localization were essential for roquin-mediated ICOS repression. However, this process did not require microRNAs or the RNA-induced silencing complex (RISC). Instead, roquin bound ICOS mRNA directly, showing an intrinsic preference for a previously unrecognized sequence in the 3′ untranslated region (3′ UTR). Our results support a model in which roquin controls ICOS expression through binding to the 3′ UTR of ICOS mRNA and by interacting with proteins that confer post-transcriptional repression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yu, D. et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007).
Vinuesa, C.G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Anderson, P., Phillips, K., Stoecklin, G. & Kedersha, N. Post-transcriptional regulation of proinflammatory proteins. J. Leukoc. Biol. 76, 42–47 (2004).
Hao, S. & Baltimore, D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat. Immunol. 10, 281–288 (2009).
Hoefig, K.P. & Heissmeyer, V. MicroRNAs grow up in the immune system. Curr. Opin. Immunol. 20, 281–287 (2008).
Stefl, R., Skrisovska, L. & Allain, F.H. RNA sequence- and shape-dependent recognition by proteins in the ribonucleoprotein particle. EMBO Rep. 6, 33–38 (2005).
Jing, Q. et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120, 623–634 (2005).
Kedde, M. et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 (2007).
Vasudevan, S. & Steitz, J.A. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 128, 1105–1118 (2007).
Tan, A.H., Wong, S.C. & Lam, K.P. Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. J. Biol. Chem. 281, 28666–28678 (2006).
Bossaller, L. et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 177, 4927–4932 (2006).
Dong, C. et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409, 97–101 (2001).
Dong, C., Temann, U.A. & Flavell, R.A. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166, 3659–3662 (2001).
McAdam, A.J. et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 165, 5035–5040 (2000).
Tafuri, A. et al. ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 (2001).
Linterman, M.A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Wan, Y.Y. et al. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA 97, 13784–13789 (2000).
Oberdoerffer, P. et al. Efficiency of RNA interference in the mouse hematopoietic system varies between cell types and developmental stages. Mol. Cell. Biol. 25, 3896–3905 (2005).
Heissmeyer, V., Ansel, K.M. & Rao, A. A plague of autoantibodies. Nat. Immunol. 6, 642–644 (2005).
Chu, C.Y. & Rana, T.M. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4, e210 (2006).
Didiot, M.C., Subramanian, M., Flatter, E., Mandel, J.L. & Moine, H. Cells lacking the fragile X mental retardation protein (FMRP) have normal RISC activity but exhibit altered stress granule assembly. Mol. Biol. Cell 20, 428–437 (2009).
Mazroui, R. et al. Trapping of messenger RNA by fragile X mental retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 11, 3007–3017 (2002).
Franks, T.M. & Lykke-Andersen, J. The control of mRNA decapping and P-body formation. Mol. Cell 32, 605–615 (2008).
Michelitsch, M.D. & Weissman, J.S. A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc. Natl. Acad. Sci. USA 97, 11910–11915 (2000).
Reijns, M.A., Alexander, R.D., Spiller, M.P. & Beggs, J.D. A role for Q/N-rich aggregation-prone regions in P-body localization. J. Cell Sci. 121, 2463–2472 (2008).
Gilks, N. et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398 (2004).
Parameswaran, P. et al. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog. 6, e1000764 (2010).
Yekta, S., Tabin, C.J. & Bartel, D.P. MicroRNAs in the Hox network: an apparent link to posterior prevalence. Nat. Rev. Genet. 9, 789–796 (2008).
Su, H., Trombly, M.I., Chen, J. & Wang, X. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev. 23, 304–317 (2009).
Athanasopoulos, V. et al. The ROQUIN family of proteins localizes to stress granules via the ROQ domain and binds target mRNAs. FEBS J. 277, 2109–2127 (2010).
Scheu, S. et al. Activation of the integrated stress response during T helper cell differentiation. Nat. Immunol. 7, 644–651 (2006).
Cougot, N., van Dijk, E., Babajko, S. & Seraphin, B. 'Cap-tabolism'. Trends Biochem. Sci. 29, 436–444 (2004).
Eulalio, A., Behm-Ansmant, I. & Izaurralde, E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8, 9–22 (2007).
Parker, R. & Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 (2007).
Lunde, B.M., Moore, C. & Varani, G. RNA-binding proteins: modular design for efficient function. Nat. Rev. Mol. Cell Biol. 8, 479–490 (2007).
Weinmann, L. et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell 136, 496–507 (2009).
Acknowledgements
We thank M. Schmidt-Supprian and D. Niessing for critical reading of the manuscript and discussions; G. Stoecklin for unpublished observations and advice; C. Vinuesa (The John Curtin School of Medical Research, Canberra, Australia) for ICOS and roquin cDNA; G. Hannon (Cold Spring Harbor Laboratory) for Dicer1fl/+ mice; C. Wilson (University of Washington) for Tg(Cd4-cre) mice; N. Kedersha and P. Anderson (Brigham and Women's Hospital) for TIA-1-knockout MEFs; U. Fischer (Biozentrum Würzburg) for anti-FMRP; H. Sarioglu for mass-spectrometry analysis; and C. Thirion and L. Behrend (Sirion Biotech) for advice and reagents for adenoviral infection. Supported by Deutsche Forschungsgemeinschaft (SFB 571 to V.H.) and Fritz Thyssen Foundation (V.H.).
Author information
Authors and Affiliations
Contributions
E.G. did most experiments, with the help of K.P.H., N.R. and C.W.; K.U.V. contributed to some experiments and edited the manuscript; L.D., E.K. and X.W. established tools and provided advice; E.G. and V.H. planned the project together; and V.H. supervised the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 6643 kb)
Rights and permissions
About this article
Cite this article
Glasmacher, E., Hoefig, K., Vogel, K. et al. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat Immunol 11, 725–733 (2010). https://doi.org/10.1038/ni.1902
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1902
This article is cited by
-
RBP–RNA interactions in the control of autoimmunity and autoinflammation
Cell Research (2023)
-
Innate immune sensing of pathogens and its post-transcriptional regulations by RNA-binding proteins
Archives of Pharmacal Research (2023)
-
Disrupting Roquin-1 interaction with Regnase-1 induces autoimmunity and enhances antitumor responses
Nature Immunology (2021)
-
Zinc finger proteins: insights into the transcriptional and post transcriptional regulation of immune response
Molecular Biology Reports (2021)
-
Roquin1 inhibits the proliferation of breast cancer cells by inducing G1/S cell cycle arrest via selectively destabilizing the mRNAs of cell cycle–promoting genes
Journal of Experimental & Clinical Cancer Research (2020)