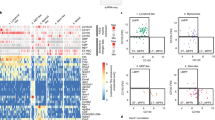
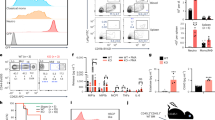
Abstract
The classical model of hematopoiesis posits the segregation of lymphoid and myeloid lineages as the earliest fate decision. The validity of this model in the mouse has been questioned; however, little is known about the lineage potential of human progenitors. Here we provide a comprehensive analysis of the human hematopoietic hierarchy by clonally mapping the developmental potential of seven progenitor classes from neonatal cord blood and adult bone marrow. Human multilymphoid progenitors, identified as a distinct population of Thy-1neg–loCD45RA+ cells in the CD34+CD38− stem cell compartment, gave rise to all lymphoid cell types, as well as monocytes, macrophages and dendritic cells, which indicated that these myeloid lineages arise in early lymphoid lineage specification. Thus, as in the mouse, human hematopoiesis does not follow a rigid model of myeloid-lymphoid segregation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Iwasaki, H. & Akashi, K. Hematopoietic developmental pathways: on cellular basis. Oncogene 26, 6687–6696 (2007).
Dick, J.E. Stem cell concepts renew cancer research. Blood 112, 4793–4807 (2008).
Kondo, M., Weissman, I.L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Adolfsson, J. et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005).
Mansson, R. et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity 26, 407–419 (2007).
Lai, A.Y. & Kondo, M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J. Exp. Med. 203, 1867–1873 (2006).
Igarashi, H., Gregory, S.C., Yokota, T., Sakaguchi, N. & Kincade, P.W. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 17, 117–130 (2002).
Martin, C.H. et al. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat. Immunol. 4, 866–873 (2003).
Lu, M., Kawamoto, H., Katsube, Y., Ikawa, T. & Katsura, Y. The common myelolymphoid progenitor: a key intermediate stage in hemopoiesis generating T and B cells. J. Immunol. 169, 3519–3525 (2002).
Katsura, Y. Redefinition of lymphoid progenitors. Nat. Rev. Immunol. 2, 127–132 (2002).
Bell, J.J. & Bhandoola, A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature 452, 764–767 (2008).
Wada, H. et al. Adult T-cell progenitors retain myeloid potential. Nature 452, 768–772 (2008).
Bhandoola, A., von Boehmer, H., Petrie, H.T. & Zuniga-Pflucker, J.C. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity 26, 678–689 (2007).
Welner, R.S., Pelayo, R. & Kincade, P.W. Evolving views on the genealogy of B cells. Nat. Rev. Immunol. 8, 95–106 (2008).
Manz, M.G., Miyamoto, T., Akashi, K. & Weissman, I.L. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl. Acad. Sci. USA 99, 11872–11877 (2002).
Galy, A., Travis, M., Cen, D. & Chen, B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity 3, 459–473 (1995).
Six, E.M. et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J. Exp. Med. 204, 3085–3093 (2007).
Hao, Q.L. et al. Identification of a novel, human multilymphoid progenitor in cord blood. Blood 97, 3683–3690 (2001).
Hoebeke, I. et al. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34+CD38−CD7+ common lymphoid progenitors expressing lymphoid-specific genes. Leukemia 21, 311–319 (2007).
Yoshikawa, Y. et al. A clonal culture assay for human cord blood lymphohematopoietic progenitors. Hum. Immunol. 60, 75–82 (1999).
Majeti, R., Park, C.Y. & Weissman, I.L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1, 635–645 (2007).
La Motte-Mohs, R.N., Herer, E. & Zuniga-Pflucker, J.C. Induction of T cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood (2004).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Delaney, C. et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 16, 232–236 (2010).
Ng, S.Y., Yoshida, T., Zhang, J. & Georgopoulos, K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity 30, 493–507 (2009).
Linton, P.J. & Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139 (2004).
Leon, B., Lopez-Bravo, M. & Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007).
Krutzik, S.R. et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11, 653–660 (2005).
Fogg, D.K. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Chicha, L., Jarrossay, D. & Manz, M.G. Clonal type I interferon-producing and dendritic cell precursors are contained in both human lymphoid and myeloid progenitor populations. J. Exp. Med. 200, 1519–1524 (2004).
Arrighi, J.F., Hauser, C., Chapuis, B., Zubler, R.H. & Kindler, V. Long-term culture of human CD34+ progenitors with FLT3-ligand, thrombopoietin, and stem cell factor induces extensive amplification of a CD34−CD14− and a CD34−CD14+ dendritic cell precursor. Blood 93, 2244–2252 (1999).
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003).
Miyamoto, T. et al. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev. Cell 3, 137–147 (2002).
Wang, H. & Morse, H.C., 3rd. IRF8 regulates myeloid and B lymphoid lineage diversification. Immunol. Res. 43, 109–117 (2009).
Pronk, C.J. et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 1, 428–442 (2007).
Hao, Q.L. et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7− lympho-myeloid thymic progenitors. Blood 111, 1318–1326 (2008).
Balciunaite, G., Ceredig, R., Massa, S. & Rolink, A.G.A. B220+CD117+CD19− hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur. J. Immunol. 35, 2019–2030 (2005).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat. Immunol. 2, 83–88 (2001).
Payne, K.J. & Crooks, G.M. Immune-cell lineage commitment: translation from mice to humans. Immunity 26, 674–677 (2007).
Vinh, D.C. et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 115, 1519–1529 (2010).
Saran, N. et al. Multiple extrathymic precursors contribute to T-cell development with different kinetics. Blood 115, 1137–1144 (2010).
van Furth, R. & Cohn, Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Auffray, C., Sieweke, M.H. & Geissmann, F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 (2009).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Kawamoto, H. & Katsura, Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 30, 193–200 (2009).
Kawamoto, H. A close developmental relationship between the lymphoid and myeloid lineages. Trends Immunol. 27, 169–175 (2006).
Melief, C.J. Cancer immunotherapy by dendritic cells. Immunity 29, 372–383 (2008).
Tacken, P.J., de Vries, I.J., Torensma, R. & Figdor, C.G. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 7, 790–802 (2007).
Acknowledgements
We thank K. Moore and the obstetrics unit of Trillium Hospital for providing cord blood samples; P.A. Penttilä, S. Zhao and L. Jamieson at the SickKids-UHN Flow Cytometry Facility for sorting; and N. Iscove for critical review of the manuscript. Supported by the Canadian Institutes for Health Research (F.N. and S.D.), the Stem Cell Network of Canadian National Centres of Excellence, the Canadian Cancer Society Research Institute, the Terry Fox Foundation, Genome Canada through the Ontario Genomics Institute, the Ontario Institute for Cancer Research, the province of Ontario, the Leukemia and Lymphoma Society, Canada Research and the Ontario Ministry of Health and Long Term Care. The views expressed here do not necessarily reflect those of the Ontario Ministry of Health and Long Term Care.
Author information
Authors and Affiliations
Contributions
S.D. and F.N. designed and did experiments; S.D. wrote the manuscript; K.E. analyzed microarray data; L.T.N. did DC population expansion experiments; and P.S.O. and J.E.D. supervised the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–5 (PDF 4132 kb)
Rights and permissions
About this article
Cite this article
Doulatov, S., Notta, F., Eppert, K. et al. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol 11, 585–593 (2010). https://doi.org/10.1038/ni.1889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1889
This article is cited by
-
Roles of Macrophages and Their Interactions with Schwann Cells After Peripheral Nerve Injury
Cellular and Molecular Neurobiology (2024)
-
Comparison of osteoclast differentiation protocols from human induced pluripotent stem cells of different tissue origins
Stem Cell Research & Therapy (2023)
-
Single cell analyses identify a highly regenerative and homogenous human CD34+ hematopoietic stem cell population
Nature Communications (2022)
-
Expansion of Human Megakaryocyte-Lineage Progeny via Aryl Hydrocarbon Receptor Antagonism with CH223191
Stem Cell Reviews and Reports (2022)
-
Differentiation Epitopes Define Hematopoietic Stem Cells and Change with Cell Cycle Passage
Stem Cell Reviews and Reports (2022)