Abstract
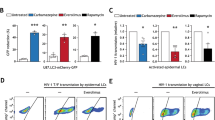
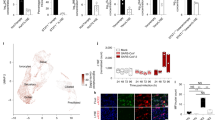
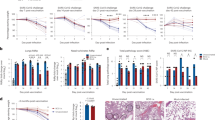
Contact-dependent communication between immune cells generates protection but also facilitates viral spread. Here we found that macrophages formed long-range actin-propelled conduits in response to negative factor (Nef), a human immunodeficiency virus type 1 (HIV-1) protein with immunosuppressive functions. Conduits attenuated immunoglobulin G2 (IgG2) and IgA class switching in systemic and intestinal lymphoid follicles by shuttling Nef from infected macrophages to B cells through a guanine-exchange factor–dependent pathway involving the amino-terminal anchor, central core and carboxy-terminal flexible loop of Nef. By showing stronger virus-specific IgG2 and IgA responses in patients with Nef-deficient virions, our data suggest that HIV-1 exploits intercellular 'highways' as a 'Trojan horse' to deliver Nef to B cells and evade humoral immunity systemically and at mucosal sites of entry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Davis, D.M. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat. Rev. Immunol. 7, 238–243 (2007).
Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010 (2004).
Sherer, N.M. & Mothes, W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 18, 414–420 (2008).
Watkins, S.C. & Salter, R.D. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 23, 309–318 (2005).
Fackler, O.T., Alcover, A. & Schwartz, O. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7, 310–317 (2007).
Sherer, N.M. et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9, 310–315 (2007).
Sowinski, S. et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10, 211–219 (2008).
Qiao, X. et al. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 7, 302–310 (2006).
Muratori, C. et al. Macrophages transmit human immunodeficiency virus type 1 products to CD4-negative cells: involvement of matrix metalloproteinase 9. J. Virol. 81, 9078–9087 (2007).
Kestler, H.W. III et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65, 651–662 (1991).
Peterlin, B.M. & Trono, D. Hide, shield and strike back: how HIV-infected cells avoid immune eradication. Nat. Rev. Immunol. 3, 97–107 (2003).
Fackler, O.T., Luo, W., Geyer, M., Alberts, A.S. & Peterlin, B.M. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3, 729–739 (1999).
Peng, B. & Robert-Guroff, M. Deletion of N-terminal myristoylation site of HIV Nef abrogates both MHC-1 and CD4 down-regulation. Immunol. Lett. 78, 195–200 (2001).
Blagoveshchenskaya, A.D., Thomas, L., Feliciangeli, S.F., Hung, C.H. & Thomas, G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111, 853–866 (2002).
Thoulouze, M.I. et al. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24, 547–561 (2006).
Burton, D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2, 706–713 (2002).
Lane, H.C. et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309, 453–458 (1983).
Moir, S. & Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 9, 235–245 (2009).
Binley, J.M. et al. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J. Virol. 71, 2799–2809 (1997).
Regulier, E.G. et al. Persistent anti-gag, -Nef, and -Rev IgM levels as markers of the impaired functions of CD4+ T-helper lymphocytes during SIVmac251 infection of cynomolgus macaques. J. Acquir. Immune Defic. Syndr. 40, 1–11 (2005).
Martinez, V. et al. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. J. Infect. Dis. 191, 2053–2063 (2005).
Schafer, F. et al. Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut 50, 608–614 (2002).
Mestecky, J. et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res. Hum. Retroviruses 20, 972–988 (2004).
De Milito, A. et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103, 2180–2186 (2004).
Brenchley, J.M., Price, D.A. & Douek, D.C. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7, 235–239 (2006).
He, B. et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 176, 3931–3941 (2006).
Swingler, S. et al. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe 4, 63–76 (2008).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 (2008).
Titanji, K. et al. Loss of memory B cells impairs maintenance of long-term serological memory during HIV-1 infection. Blood 108, 1580–1587 (2006).
Hart, M. et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J. Immunol. 178, 8212–8220 (2007).
Moir, S. et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc. Natl. Acad. Sci. USA 100, 6057–6062 (2003).
Poudrier, J. et al. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-gamma and IL-6. Immunity 15, 173–185 (2001).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Xu, W. et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8, 294–303 (2007).
Klein, U. et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 7, 773–782 (2006).
Shaffer, A.L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Greenberg, M.E., Iafrate, A.J. & Skowronski, J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17, 2777–2789 (1998).
Aiken, C., Konner, J., Landau, N.R., Lenburg, M.E. & Trono, D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76, 853–864 (1994).
Greenberg, M.E. et al. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16, 6964–6976 (1997).
Piguet, V. et al. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the μ chain of adaptor complexes. EMBO J. 17, 2472–2481 (1998).
Greenberg, M., DeTulleo, L., Rapoport, I., Skowronski, J. & Kirchhausen, T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8, 1239–1242 (1998).
Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Rodriguez, B. et al. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS 17, 1983–1985 (2003).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Dyer, W.B. et al. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J. Virol. 73, 436–443 (1999).
Burton, D.R. et al. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5, 233–236 (2004).
Popovic, M. et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 102, 14807–14812 (2005).
Prost, S. et al. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARγ/STAT5 signaling pathway in macaques. J. Clin. Invest. 118, 1765–1775 (2008).
Schrofelbauer, B., Yu, Q., Zeitlin, S.G. & Landau, N.R. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 79, 10978–10987 (2005).
Santa-Marta, M., Aires da Silva, F., Fonseca, A.M., Rato, S. & Goncalves, J. HIV-1 Vif protein blocks the cytidine deaminase activity of B-cell specific AID in E. coli by a similar mechanism of action. Mol. Immunol. 44, 583–590 (2007).
Begum, N.A. et al. Requirement of non-canonical activity of uracil DNA glycosylase for class switch recombination. J. Biol. Chem. 282, 731–742 (2007).
Mann, J. et al. Functional analysis of HIV type 1 Nef reveals a role for PAK2 as a regulator of cell phenotype and function in the murine dendritic cell line, DC2.4. J. Immunol. 175, 6560–6569 (2005).
Conboy, I.M., Manoli, D., Mhaiskar, V. & Jones, P.P. Calcineurin and vacuolar-type H+-ATPase modulate macrophage effector functions. Proc. Natl. Acad. Sci. USA 96, 6324–6329 (1999).
Wiley, R.D. & Gummuluru, S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA 103, 738–743 (2006).
Costa, L.J. et al. Interactions between Nef and AIP1 proliferate multivesicular bodies and facilitate egress of HIV-1. Retrovirology 3, 33 (2006).
Fujii, Y., Otake, K., Tashiro, M. & Adachi, A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 393, 93–96 (1996).
Acknowledgements
We thank M. Stevenson (University of Massachusetts Medical School) for the ΔNef-ADA plasmid; B. Berkhout (Academic Medical Center) for the ΔNef-LAI plasmid; S.J. Burakoff (New York University) for the Nef-dsRED vector; M.G. Caron, (Duke University) for dominant negative dynamin-K44A and β-arrestin-2–V54D; J.G. Donaldson (National Institutes of Health) for dominant negative ARF6-T27N; Y. Zheng (Cincinnati Children's Hospital Medical Center) for dominant negative RhoA-N19, Cdc42-N17 and Rac1-N17; P. Marignani (Dalhousie University) for dominant negative Vav2-R/S; J.P. Moore (Weill Medical College of Cornell University) for the ΔNef-HIV-1–expressing NL4-3/9-7-dsRed plasmid; A. Pernis (Columbia University) for reagents; and all reagent donors for discussions. Supported by the US National Institutes of Health (AI07621 to W.X.; and R01 AI057653, R01 AI057653-S1 and R01 AI074378 to A.Ce.), The Irma T. Hirschl Charitable Trust (to A.Ce.), the Cornell Comprehensive Cancer Center (Chronic Lymphocytic Leukemia Research Center Award; to A.Ce.), the Ministerio de Ciencia e Innovación (Plan Nacional de Investigación Cientifica, Desarollo e Innovación Tecnológica SAF 2008-02725 to A.Ce.) and the Cancer Research Institute (to P.A.S.).
Author information
Authors and Affiliations
Contributions
W.X. and P.A.S. designed and did research; B.H and K.C. did research and discussed data; J.S.S., W.B.D., A.Cha., D.M.K. and A.Chi. provided samples and discussed data; M.S., T.J.K. and R.W.S. provided reagents and did research; S.C.B. provided clinical data; L.C.-G. did electron microscopy; and A.Ce. designed research, discussed data and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15, Supplementary Tables 1–2 and Supplementary Methods (PDF 3542 kb)
Supplementary Movie 1
Membrane ruffling and protrusions in Nef-containing macrophage-like cells. Three-dimensional animation of a THP-1 macrophage-like cell expressing Nef-eGFP. The movie was generated by acquiring up to 15 XY planes with 0.4 ∼ 0.5 μm Z spacing by confocal microscopy. Three-dimensional views were constructed with maximum projection and exported as 30-40 tiff images. QuickTime Pro software was used to edit images into movies. One of several experiments yielding similar results. (MOV 1681 kb)
Supplementary Movie 2
Nef-containing macrophage-like cells form short-range intercellular bridges. Three-dimensional animation of two THP-1 macrophage-like cells expressing Nef-eGFP. One of several experiments yielding similar results. (MOV 2355 kb)
Supplementary Movie 3
Macrophage-like cells can transfer cytoplasmic material to B cells through both short- and long-range intercellular mechanisms upon activation. Time-lapse animation of macrophage-like THP-1 cells pre-loaded with LysoTracker (green) and co-cultured with IgD+ B cells in the presence of LPS, a microbial product with macrophage- but not B cell-stimulating activity. Live-cell DIC and epifluores cence images were acquired every 20 sec to generate this time-lapse movie. One of 5 experiments yielding similar results. (MOV 4555 kb)
Supplementary Movie 4
HIV-1-infected primary macrophages form long-range Nef-trafficking intercellular conduits. Three-dimensional animation of two primary macrophages infected with HIV-1 ADA and stained for Nef (red) in the presence of the membrane-specific lectin WGA (green). One of several experiments yielding similar results. (MOV 2257 kb)
Rights and permissions
About this article
Cite this article
Xu, W., Santini, P., Sullivan, J. et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol 10, 1008–1017 (2009). https://doi.org/10.1038/ni.1753
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1753
This article is cited by
-
M-Sec facilitates intercellular transmission of HIV-1 through multiple mechanisms
Retrovirology (2020)
-
HIV Nef enhances the expression of oncogenic c-MYC and activation-induced cytidine deaminase in Burkitt lymphoma cells, promoting genomic instability
Infectious Agents and Cancer (2020)
-
Myosin-X is essential to the intercellular spread of HIV-1 Nef through tunneling nanotubes
Journal of Cell Communication and Signaling (2019)
-
Rapid decrease in titer and breadth of neutralizing anti-HCV antibodies in HIV/HCV-coinfected patients who achieved SVR
Scientific Reports (2019)
-
Perspective on nanochannels as cellular mediators in different disease conditions
Cell Communication and Signaling (2018)