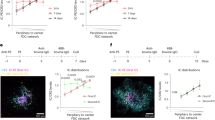
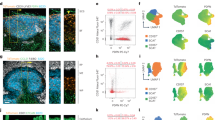
Abstract
Subcapsular sinus (SCS) macrophages capture antigens from lymph and present them intact for B cell encounter and follicular delivery. However, the properties of SCS macrophages are poorly defined. Here we show SCS macrophage development depended on lymphotoxin-α1β2, and the cells had low lysosomal enzyme expression and retained opsonized antigens on their surface. Intravital imaging revealed immune complexes moving along macrophage processes into the follicle. Moreover, noncognate B cells relayed antigen opsonized by newly produced antibodies from the subcapsular region to the germinal center, and affinity maturation was impaired when this transport process was disrupted. Thus, we characterize SCS macrophages as specialized antigen-presenting cells functioning at the apex of an antigen transport chain that promotes humoral immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Nossal, G.J., Abbot, A., Mitchell, J. & Lummus, Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J. Exp. Med. 127, 277–290 (1968).
Fossum, S. The architecture of rat lymph nodes. IV. Distribution of ferritin and colloidal carbon in the draining lymph nodes after foot-pad injection. Scand. J. Immunol. 12, 433–441 (1980).
Szakal, A.K., Holmes, K.L. & Tew, J.G. Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J. Immunol. 131, 1714–1727 (1983).
Carrasco, Y.R. & Batista, F.D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007).
Phan, T.G., Grigorova, I., Okada, T. & Cyster, J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8, 992–1000 (2007).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Steinman, R.M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
MacLennan, I.C.M. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Allen, C.D., Okada, T. & Cyster, J.G. Germinal-center organization and cellular dynamics. Immunity 27, 190–202 (2007).
Schwickert, T.A. et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87 (2007).
Allen, C.D., Okada, T., Tang, H.L. & Cyster, J.G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007).
Crocker, P.R. & Gordon, S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med. 164, 1862–1875 (1986).
Hume, D.A., Robinson, A.P., MacPherson, G.G. & Gordon, S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J. Exp. Med. 158, 1522–1536 (1983).
Fu, Y.-X. & Chaplin, D.D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17, 399–433 (1999).
Browning, J.L. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol. Rev. 223, 202–220 (2008).
Ngo, V.N., Cornall, R.J. & Cyster, J.G. Splenic T zone development is B cell dependent. J. Exp. Med. 194, 1649–1660 (2001).
Cascalho, M., Ma, A., Lee, S., Masat, L. & Wabl, M. A quasi-monoclonal mouse. Science 272, 1649–1652 (1996).
Germain, R.N., Miller, M.J., Dustin, M.L. & Nussenzweig, M.C. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat. Rev. Immunol. 6, 497–507 (2006).
Roozendaal, R. & Carroll, M.C. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 219, 157–166 (2007).
Chtanova, T. et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29, 487–496 (2008).
Farr, A.G., Cho, Y. & De Bruyn, P.P. The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am. J. Anat. 157, 265–284 (1980).
Prigozhina, N.L. & Waterman-Storer, C.M. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr. Biol. 14, 88–98 (2004).
Wulfing, C. & Davis, M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Moss, W.C., Irvine, D.J., Davis, M.M. & Krummel, M.F. Quantifying signaling-induced reorientation of T cell receptors during immunological synapse formation. Proc. Natl. Acad. Sci. USA 99, 15024–15029 (2002).
Engstler, M. et al. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 131, 505–515 (2007).
Kabashima, K. et al. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 (2005).
Wang, Y. et al. Antigen persistence is required for somatic mutation and affinity maturation of immunoglobulin. Eur. J. Immunol. 30, 2226–2234 (2000).
Hannum, L.G., Haberman, A.M., Anderson, S.M. & Shlomchik, M.J. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J. Exp. Med. 192, 931–942 (2000).
Brady, L.J. Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect. Immun. 73, 671–678 (2005).
Getahun, A. & Heyman, B. How antibodies act as natural adjuvants. Immunol. Lett. 104, 38–45 (2006).
Song, H., Nie, X., Basu, S. & Cerny, J. Antibody feedback and somatic mutation in B cells: regulation of mutation by immune complexes with IgG antibody. Immunol. Rev. 162, 211–218 (1998).
Ehrenstein, M.R., O'Keefe, T.L., Davies, S.L. & Neuberger, M.S. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA 95, 10089–10093 (1998).
Boes, M. et al. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160, 4776–4787 (1998).
Hadjantonakis, A.K., Macmaster, S. & Nagy, A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2, 11 (2002).
Schaefer, B.C., Schaefer, M.L., Kappler, J.W., Marrack, P. & Kedl, R.M. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell. Immunol. 214, 110–122 (2001).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350, 423–426 (1991).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M.H. & Pfeffer, K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Molina, H. et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA 93, 3357–3361 (1996).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Crocker, P.R. & Gordon, S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J. Exp. Med. 169, 1333–1346 (1989).
Paus, D. et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 203, 1081–1091 (2006).
Carroll, R.C. et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 96, 14112–14117 (1999).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002).
Valledor, A.F., Borras, F.E., Cullell-Young, M. & Celada, A. Transcription factors that regulate monocyte/macrophage differentiation. J. Leukoc. Biol. 63, 405–417 (1998).
Phan, T.G. et al. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J. Exp. Med. 197, 845–860 (2003).
Smith-Gill, S.J., Mainhart, C.R., Lavoie, T.B., Rudikoff, S. & Potter, M. VL-VH expression by monoclonal antibodies recognizing avian lysozyme. J. Immunol. 132, 963–967 (1984).
Smith-Gill, S.J., Lavoie, T.B. & Mainhart, C.R. Antigenic regions defined by monoclonal antibodies correspond to structural domains of avian lysozyme. J. Immunol. 133, 384–393 (1984).
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150 (2005).
Acknowledgements
We thank L. Shiow for cell sorting and discussions, K. Suzuki for preparation of HEL-PE, C. Allen for help with single cell sorting, I. Girgorova for help with video editing, J. An for mouse screening, J. Atkinson (Washington University) for CR2-deficient mice, M. Wabl (University of California, San Francisco) for QM mice, J. Browning (Biogen Idec) for LTβR-Fc, P. Crocker (University of Glasgow) for Ser-4 antibody, A. Bullen and M. Krummel for help with the two-photon microscope, A. Bankovich for advice, the UCSF Hybridoma Core, the Gladstone Genomics Core and the Diabetes and Endocrinology Research Center Microscopy Core and Biological Imaging Development Center. Supported by an Australian National Health and Medical Research Council CJ Martin fellowship (T.G.P.), a US National Science Foundation graduate research fellowship (J.A.G.), a Howard Hughes Medical Institute investigator award (J.G.C.), the US National Institutes of Health (AI45073 and AI40098) and a Sandler New Technology Award.
Author information
Authors and Affiliations
Contributions
T.G.P. and J.G.C. designed and conceptualized the research; T.G.P., J.A.G. and E.E.G. did the experiments; Y.X. performed the microarray and Q-PCR analysis; T.G.P., J.A.G., E.E.G. and J.G.C. analyzed the data; T.G.P., J.A.G. and E.E.G. prepared figures; T.G.P. and J.G.C. wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 401 kb)
Supplementary Video 1
SCS macrophages transport immune complexes along cellular processes into the follicle. (MPG 2404 kb)
Supplementary Video 2
Noncognate B cells carry immune complex from subcapsular region into the GC. (MPG 5312 kb)
Supplementary Video 3
Noncognate B cell carrying immune complex in the GC. (MPG 5212 kb)
Supplementary Video 4
Noncognate B cell migrating in GC light zone with immune complex. (MPG 7836 kb)
Rights and permissions
About this article
Cite this article
Phan, T., Green, J., Gray, E. et al. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 10, 786–793 (2009). https://doi.org/10.1038/ni.1745
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1745
This article is cited by
-
Recreation of an antigen-driven germinal center in vitro by providing B cells with phagocytic antigen
Communications Biology (2023)
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
-
Recycling of memory B cells between germinal center and lymph node subcapsular sinus supports affinity maturation to antigenic drift
Nature Communications (2022)
-
Delivery of nanovaccine towards lymphoid organs: recent strategies in enhancing cancer immunotherapy
Journal of Nanobiotechnology (2021)
-
B cell depletion therapies in autoimmune disease: advances and mechanistic insights
Nature Reviews Drug Discovery (2021)