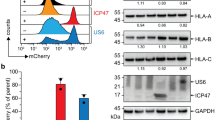
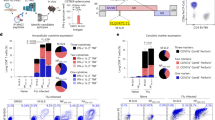
Abstract
Although cytotoxic T lymphocytes (CTLs) in people infected with human immunodeficiency virus type 1 can potentially target multiple virus epitopes, the same few are recognized repeatedly. We show here that CTL immunodominance in regions of the human immunodeficiency virus type 1 group-associated antigen proteins p17 and p24 correlated with epitope abundance, which was strongly influenced by proteasomal digestion profiles, affinity for the transporter protein TAP, and trimming mediated by the endoplasmatic reticulum aminopeptidase ERAAP, and was moderately influenced by HLA affinity. Structural and functional analyses demonstrated that proteasomal cleavage 'preferences' modulated the number and length of epitope-containing peptides, thereby affecting the response avidity and clonality of T cells. Cleavage patterns were affected by both flanking and intraepitope CTL-escape mutations. Our analyses show that antigen processing shapes CTL response hierarchies and that viral evolution modifies cleavage patterns and suggest strategies for in vitro vaccine optimization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yewdell, J.W. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity 25, 533–543 (2006).
Altfeld, M. et al. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med. 3, e403 (2006).
Bihl, F. et al. Impact of HLA-B alleles, epitope binding affinity, functional avidity, and viral coinfection on the immunodominance of virus-specific CTL responses. J. Immunol. 176, 4094–4101 (2006).
Goulder, P.J. et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193, 181–194 (2001).
Chen, W., Anton, L.C., Bennink, J.R. & Yewdell, J.W. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12, 83–93 (2000).
Kloetzel, P.M. Antigen processing by the proteasome. Nat. Rev. Mol. Cell Biol. 2, 179–187 (2001).
Tenzer, S. et al. Quantitative analysis of prion-protein degradation by constitutive and immuno-20S proteasomes indicates differences correlated with disease susceptibility. J. Immunol. 172, 1083–1091 (2004).
Toes, R.E. et al. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J. Exp. Med. 194, 1–12 (2001).
Van den Eynde, B.J. & Morel, S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Curr. Opin. Immunol. 13, 147–153 (2001).
HIV Molecular Immunology 2006/2007 (eds. Korber, B.T.M. et al.) 53–248 (Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, New Mexico, 2006–2007).
Iversen, A.K. et al. Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nat. Immunol. 7, 179–189 (2006).
Goulder, P.J. & Watkins, D.I. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8, 619–630 (2008).
Kaslow, R.A. et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2, 405–411 (1996).
Goulder, P.J. et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3, 212–217 (1997).
Schneidewind, A. et al. Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J. Virol. 82, 5594–5605 (2008).
Schneidewind, A. et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J. Virol. 81, 12382–12393 (2007).
Draenert, R. et al. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J. Exp. Med. 199, 905–915 (2004).
Milicic, A. et al. CD8+ T cell epitope-flanking mutations disrupt proteasomal processing of HIV-1 Nef. J. Immunol. 175, 4618–4626 (2005).
Zimbwa, P. et al. Precise identification of a human immunodeficiency virus type 1 antigen processing mutant. J. Virol. 81, 2031–2038 (2007).
Le Gall, S., Stamegna, P. & Walker, B.D. Portable flanking sequences modulate CTL epitope processing. J. Clin. Invest. 117, 3563–3575 (2007).
Leslie, A. et al. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J. Exp. Med. 201, 891–902 (2005).
Ossendorp, F. et al. A single residue exchange within a viral CTL epitope alters proteasome-mediated degradation resulting in lack of antigen presentation. Immunity 5, 115–124 (1996).
Shimbara, N. et al. Contribution of proline residue for efficient production of MHC class I ligands by proteasomes. J. Biol. Chem. 273, 23062–23071 (1998).
Fruci, D., Niedermann, G., Butler, R.H. & van Endert, P.M. Efficient MHC class I-independent amino-terminal trimming of epitope precursor peptides in the endoplasmic reticulum. Immunity 15, 467–476 (2001).
Gubler, B. et al. Substrate selection by transporters associated with antigen processing occurs during peptide binding to TAP. Mol. Immunol. 35, 427–433 (1998).
van Endert, P.M. et al. The peptide-binding motif for the human transporter associated with antigen processing. J. Exp. Med. 182, 1883–1895 (1995).
Saric, T. et al. An IFN-γ-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I–presented peptides. Nat. Immunol. 3, 1169–1176 (2002).
Serwold, T., Gonzalez, F., Kim, J., Jacob, R. & Shastri, N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419, 480–483 (2002).
Altfeld, M. et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193, 169–180 (2001).
Lee, J.K. et al. T cell cross-reactivity and conformational changes during TCR engagement. J. Exp. Med. 200, 1455–1466 (2004).
Martinez-Hackert, E. et al. Structural basis for degenerate recognition of natural HIV peptide variants by cytotoxic lymphocytes. J. Biol. Chem. 281, 20205–20212 (2006).
Streeck, H. et al. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 81, 7725–7731 (2007).
Bouillot, M. et al. Physical association between MHC class I molecules and immunogenic peptides. Nature 339, 473–475 (1989).
Huet, S. et al. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int. Immunol. 2, 311–316 (1990).
Jardetzky, T.S., Lane, W.S., Robinson, R.A., Madden, D.R. & Wiley, D.C. Identification of self peptides bound to purified HLA-B27. Nature 353, 326–329 (1991).
Nixon, D.F. et al. HIV-1 Gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336, 484–487 (1988).
Urban, R.G. et al. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc. Natl. Acad. Sci. USA 91, 1534–1538 (1994).
Betts, M.R. et al. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74, 9144–9151 (2000).
Altfeld, M.A. et al. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 74, 8541–8549 (2000).
Streeck, H. et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5, e100 (2008).
Almeida, J.R. et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204, 2473–2485 (2007).
Wearsch, P.A. & Cresswell, P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat. Immunol. 8, 873–881 (2007).
Brumme, Z.L. et al. Evidence of differential HLA class I-mediated viral evolution in functional and accessory/regulatory genes of HIV-1. PLoS Pathog. 3, e94 (2007).
Goulder, P.J. & Watkins, D.I. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4, 630–640 (2004).
Goulder, P.J. et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412, 334–338 (2001).
Collins, E.J., Garboczi, D.N. & Wiley, D.C. Three-dimensional structure of a peptide extending from one end of a class I MHC binding site. Nature 371, 626–629 (1994).
Wilson, J.D. et al. Oligoclonal expansions of CD8+ T cells in chronic HIV infection are antigen specific. J. Exp. Med. 188, 785–790 (1998).
Gao, X. et al. AIDS restriction HLA allotypes target distinct intervals of HIV-1 pathogenesis. Nat. Med. 11, 1290–1292 (2005).
Burgevin, A. et al. A detailed analysis of the murine TAP transporter substrate specificity. PLoS ONE 3, e2402 (2008).
Sylvester-Hvid, C. et al. Establishment of a quantitative ELISA capable of determining peptide-MHC class I interaction. Tissue Antigens 59, 251–258 (2002).
Acknowledgements
We thank the patients for donating samples; B. Baadegaard and L.P. Jensen for patient management; D. Hass, T. Rostron, J. Frankland and J. Forsch for technical assistance; and N. Willcox for discussions. Supported by the Novo Nordisk Foundation, the Danish AIDS foundation, the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 490, E6, Z3), the Genomes2Vaccines Specific Targeted Research Project, Sixth Framework Programme (LSHB-CT-2003-503231), the Hochschulbauförderungsgesetz Program (HBFG-122-605), the Forschungszentrum Immunologie at the University of Mainz, the Nuffield Dominions Trust, Cancer Research UK, the European Union (LSHG-CT-2006-031220, LSHC-CT-2006-518234 and HEALTH-2007-222773), the Wellcome Trust, The James Martin 21st Century School at the University of Oxford, the National Institute for Health Research Biomedical Research Centre Programme, and the UK Medical Research Council.
Author information
Authors and Affiliations
Contributions
A.K.N.I. conceived and designed the overall study and wrote the manuscript; S.T., H.S., S.B., P.v.E. and A.K.N.I. planned and supervised experiments; S.T., E.W., A.B., G.S.-J., L.F., K.L., C.-h.C., M.H., M.W., N.A. and A.K.N.I. did experiments; S.T., H.S., S.B., G.S.-J., E.Y.J., P.K., P.v.E. and A.K.N.I. analyzed data; J.G. and A.K.N.I. provided patient samples; S.B., P.v.E., A.J.M., L.F., A.K.N.I. and H.S. provided reagents; and S.T., S.B., H.S., P.K., L.F., G.S.-J., E.Y.J. and P.v.E. contributed intellectual input.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–3 (PDF 5449 kb)
Rights and permissions
About this article
Cite this article
Tenzer, S., Wee, E., Burgevin, A. et al. Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat Immunol 10, 636–646 (2009). https://doi.org/10.1038/ni.1728
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1728
This article is cited by
-
Protein degradation by human 20S proteasomes elucidates the interplay between peptide hydrolysis and splicing
Nature Communications (2024)
-
InvitroSPI and a large database of proteasome-generated spliced and non-spliced peptides
Scientific Data (2023)
-
Large database for the analysis and prediction of spliced and non-spliced peptide generation by proteasomes
Scientific Data (2020)
-
MHC-I peptides get out of the groove and enable a novel mechanism of HIV-1 escape
Nature Structural & Molecular Biology (2017)
-
Host genotype and time dependent antigen presentation of viral peptides: predictions from theory
Scientific Reports (2017)