Abstract
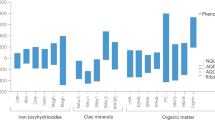
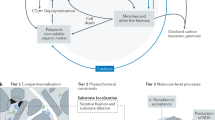
The decay of soil and sedimentary organic matter yieldsorganic compounds with a high molecular weight, termed humic substances1. Microorganisms can transfer electrons to dissolved humic substances2, and reduced humic substances can rapidly reduce iron(III) oxides3. Thus, dissolved humic substances can serve as electron shuttles that promote iron(III) oxide reduction in sediments2,4. However, most humic substances in soils and sediments are in particulate, rather than dissolved, form1; the ability of microorganisms to reduce solid-phase humics and their capacity to shuttle electrons is thus far unknown. Here we show through incubation experiments and electron spin resonance measurements that iron(III)-oxide-reducing bacteria can transfer electrons to solid-phase humic substances in sediments sampled from wetlands. Although the electron-accepting capacity of the solid-phase humics was modest, solid-phase humics significantly accelerated iron(III) oxide reduction, by shuttling electrons from bacteria to oxide surfaces. Microbial solid-phase humics reduction represents a new mechanism for extracellular electron transfer that can facilitate reduction of iron(III) oxide and other redox reactions in sediments and soils.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stevenson, F. J. Humus Chemistry: Genesis, Composition, Reactions (John Wiley, 1994).
Lovley, D. R., Coates, J. D., Blunt-Harris, E. L., Phillips, E. J. P. & Woodward, J. C. Humic substances as electron acceptors for microbial respiration. Nature 382, 445–448 (1996).
Skogerboe, R. K. & Wilson, S. A. Reduction of ionic species by fulvic acid. Anal. Chem. 53, 228–232 (1981).
Nevin, K. P. & Lovley, D. R. Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Environ. Sci. Technol. 34, 2472–2478 (2000).
Lovley, D. R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55, 259–287 (1991).
Thamdrup, B. Bacterial manganese and iron reduction in aquatic sediments. Adv. Microb. Ecol. 16, 41–84 (2000).
Kappler, A., Benz, M., Schink, B. & Brune, A. Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 47, 85–92 (2004).
Szilagyi, M. Redox properties and determination of normal potential of peat-water system. Soil Sci. Am. J. 115, 434–437 (1973).
Van der Zee, F. P., Bisschops, I. A. E., Lettinga, G. & Field, J. A. Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environ. Sci. Technol. 37, 402–408 (2003).
Lovley, D. R. & Phillips, E. J. P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51, 683–689 (1986).
Scott, D. T., McKnight, D. M., Blunt-Harris, E. L., Kolesar, S. E. & Lovley, D. R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 2894, 2984–2989 (1998).
Paul, A. et al. Nature and abundance of organic radicals in natural organic matter: Effect of pH and irradiation. Environ. Sci. Technol. 40, 5897–5903 (2006).
Stoesser, R., Bartoll, J., Schirrmeister, L., Ernst, R. & Lueck, R. ESR of trapped holes and electrons in natural and synthetic carbonates, silicates and aluminosilicates. Appl. Radiat. Isot. 47, 1489–1496 (1996).
Jiang, J., Bauer, I., Paul, A. & Kappler, A. Arsenic redox changes by microbially and chemically formed semiquinone radicals and hydroquinones in a humic substance model quinone. Environ. Sci. Technol. 43, 3639–3645 (2009).
Ratasuk, N. & Nanny, M. A. Characterization and quantification of reversible redox sites in humic substances. Environ. Sci. Technol. 41, 7844–7850 (2007).
Jiang, J. & Kappler, A. Kinetics of microbial and chemical reduction of humic substances: Implications for electron shuttling. Environ. Sci. Technol. 42, 3563–3569 (2008).
Heitmann, T., Goldhammer, T., Beer, J. & Blodau, C. Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Global. Change Biol. 13, 1771–1785 (2007).
Segers, R. & Kengen, S. W. M. Soil methane production as a function of anaerobic carbon mineralization: A process model. Soil Biol. Biochem. 30, 1107–1117 (1998).
Neubauer, S. C., Givler, K., Valentine, S. K. & Megonigal, J. P. Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86, 3334–3344 (2005).
Van der Zee, F. R. & Cervantes, F. J. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol. Adv. 27, 256–277 (2009).
Gu, B. & Chen, J. Enhanced microbial reduction of Cr(VI) and U(VI) by different natural organic matter fractions. Geochim. Cosmochim. Acta 67, 3575–3582 (2003).
Collins, R. & Picardal, F. Enhanced anaerobic transformations of carbon tetrachloride by soil organic matter. Environ. Toxicol. Chem. 18, 2703–2710 (1999).
Luu, Y., Ramsay, B. A. & Ramsay, J. A. Nitrilotriacetate stimulation of anaerobic Fe(III) respiration by mobilization of humic materials in soil. Appl. Environ. Microbiol. 69, 5255–5262 (2003).
Myers, J. M. & Myers, C. R. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 67, 260–269 (2001).
Poulton, S. W. & Raiswell, R. Chemical and physical characteristics of iron oxides in riverine and glacial meltwater sediments. Chem. Geol. 218, 203–221 (2005).
Wagai, R. & Mayer, L. M. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Acta 71, 25–35 (2007).
Scheinost, A. C., Kretzschmar, R., Christl, I. & Jacobsen, C. in Humic Substances Structures, Models, and Functions (eds Ghabbour, E. A. & Davies, G.) 39–50 (The Royal Society of Chemistry, 2001).
Nielsen, L. P., Risgaard-Petersen, N., Fossing, H., Christensen, P. B. & Sayama, M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463, 1071–1074 (2010).
Reguera, G. et al. Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101 (2005).
Gorby, Y. A. et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis MR-1 and other microorganisms. Proc. Natl Acad. Sci. USA 103, 11358–11363 (2006).
Acknowledgements
This research was financially supported by grants from the US Department of Energy (Office of Biological and Environmental Research) and the US National Science Foundation (Directorates for Biological Sciences and Geosciences) to E.E.R.; by an IPSWaT fellowship from the German Federal Ministry for Science and Education to J.J.; by financial support from the German Research Foundation to A.K. and A.P. and financial support from the Stifterverband der Wissenschaft to A.K. We thank G. Scott, B. Golson and B. Kollah for technical assistance, and the late R. G. Wetzel for fruitful early discussions of microbial humics transformation. This letter is dedicated to the memory of R. G. Wetzel.
Author information
Authors and Affiliations
Contributions
The concept of HSsolid reduction was developed together by E.E.R. and A.K. E.E.R. coordinated the overall project, conducted or directed microbial and chemical reduction assays, analysed data and wrote the paper. A.K. directed the S. oneidensis reduction assays, coordinated with A.P. and R.S. on the ESR analyses and wrote the paper. I.B. and J.J. conducted the S. oneidensis microbial reduction assays, and I.B. helped to coordinate the ESR analyses. A.P. and R.S. conducted and interpreted the ESR analyses; H.K. and H.X. conducted and interpreted the TEM analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 466 kb)
Rights and permissions
About this article
Cite this article
Roden, E., Kappler, A., Bauer, I. et al. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nature Geosci 3, 417–421 (2010). https://doi.org/10.1038/ngeo870
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo870
This article is cited by
-
Dissolved organic matter (DOM) enhances the competitiveness of weak exoelectrogens in a soil electroactive biofilm
Carbon Research (2024)
-
Electromicrobiology: the ecophysiology of phylogenetically diverse electroactive microorganisms
Nature Reviews Microbiology (2022)
-
Suppressing peatland methane production by electron snorkeling through pyrogenic carbon in controlled laboratory incubations
Nature Communications (2021)
-
An evolving view on biogeochemical cycling of iron
Nature Reviews Microbiology (2021)
-
A review on metal oxide (FeOx/MnOx) mediated nitrogen removal processes and its application in wastewater treatment
Reviews in Environmental Science and Bio/Technology (2021)