Abstract
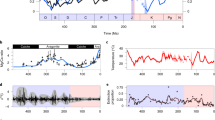
Calcareous nannoplankton, a large group of marine autotrophs that produce carbonate skeletons, were decimated to less than 10% of species during the Cretaceous/Palaeogene boundary mass extinction, 65 million years ago. Although the mass extinction followed an impact event, the exact cause of the nannoplankton mortality is not well understood. Here we assess the timing and spatial variability of nannoplankton extinction by analysing nannofossil counts in Cretaceous/Palaeogene boundary sections from all of the main ocean basins. We find that extinction rates were higher in the Northern Hemisphere oceans, and diversity remained low for 310,000 years. In contrast, Southern Hemisphere oceans showed lower extinction rates, and a nearly immediate recovery of normal nannoplankton populations. We propose that the oblique, northward impact concentrated ejected particulates into the Northern Hemisphere, blocking sunlight and suppressing photosynthesis. Increased rates of extinction and a prolonged recovery would then be associated with the greatest concentration of particulates. We speculate that metal poisoning from fallout of the particulates may have exacerbated and extended the nannoplankton crisis in the Northern Hemisphere, and thereby slowed the recovery of the Northern Hemisphere marine food web.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Raup, D. M. & Sepkoski, J. J. Mass extinctions in the marine fossil record. Science 215, 1501–1503 (1982).
Erwin, D. H. Lessons from the past: Biotic recoveries from mass extinctions. Proc. Natl Acad. Sci. USA 98, 5399–5403 (2001).
Thierstein, H. R. Terminal Cretaceous plankton extinctions: A critical assessment. Geol. Soc. Am. Special Paper 190, 385–399 (1982).
Sheehan, P. M. & Fastovsky, D. E. Major extinctions of land-dwelling vertebrates at the Cretaceous–Tertiary boundary, eastern Montana. Geology 20, 556–560 (1992).
Nichols, D. J. & Johnson, K. R. Plants and the K–T Boundary (Cambridge Paleobiology Series, 2008).
Kring, D. A. The Chicxulub impact event and its environmental consequences at the Cretaceous–Tertiary boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 255, 4–21 (2007).
Keller, G., Adatte, T., Gardin, S., Bartolini, A. & Bajpai, S. Main Deccan volcanism phase ends near the K–T boundary; Evidence from the Krishna–Godavari Basin, SE India. Earth Planet. Sci. Lett. 268, 293–311 (2008).
Robinson, N., Ravizza, G., Coccioni, R., Peucker-Ehrenbrink, B. & Norris, R. A high-resolution marine 187Os/188Os record for the late Maastrichtian: Distinguishing the chemical fingerprints of Deccan volcanism and the KP impact event. Earth Planet. Sci. Lett. 281, 159–168 (2009).
Alvarez, L. W., Alvarez, W., Asaro, F. & Michel, H. V. Extraterrestrial cause for the Cretaceous–Tertiary extinction. Science 208, 1095–1108 (1980).
Griffis, K. & Chapman, D. J. Survival of phytoplankton under prolonged darkness; implications for the Cretaceous–Tertiary boundary darkness hypothesis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 67, 305–314 (1988).
Erickson, D. J. & Dickson, S. M. Global trace-element biogeochemistry at the K/Pg boundary; oceanic and biotic response to a hypothetical meteorite impact. Geology 15, 1014–1017 (1987).
D’Hondt, S., Pilson, M. E. Q., Sigurdsson, H., Hanson, A. K. Jr & Carey, S. Surface-water acidification and extinction at the Cretaceous–Tertiary boundary. Geology 22, 983–986 (1994).
Toon, O. B., Zahnle, K., Morrison, D., Turco, R. P. & Covey, C. Environmental perturbations caused by the impacts of asteroids and comets. Rev. Geophys. 35, 41–78 (1997).
Sigurdsson, H., D’Hondt, S. & Carey, S. The impact of the Cretaceous/Tertiary bolide on evaporite terrane and generation of major sulfuric acid aerosol. Earth Planet. Sci. Lett. 109, 543–559 (1992).
Raup, D. M. & Jablonski, D. Geography of end-Cretaceous marine bivalve extinctions. Science 260, 971–973 (1993).
Jablonski, D. & Raup, D. M. Selectivity of end-Cretaceous marine bivalve extinctions. Science 268, 389–391 (1995).
Sole, R. V., Montoya, J. M. & Erwin, D. H. Recovery after mass extinction; evolutionary assembly in large-scale biosphere dynamics. Phil. Trans. R. Soc. Lond. 357, 697–707 (2002).
Bown, P. R., Lees, J. A. & Young, J. R. in Coccolithophores; From Molecular Processes to Global Impact (eds Thierstein, H. R. & Young, J. R.) 481–508 (Springer, 2004).
Coxall, H. K., D’Hondt, S. & Zachos, J. C. Pelagic evolution and environmental recovery after the Cretaceous–Paleogene mass extinction. Geology 34, 297–300 (2006).
Zachos, J. C., Arthur, M. A. & Dean, W. E. Geochemical evidence for suppression of pelagic marine productivity at the Cretaceous/Tertiary boundary. Nature 337, 61–64 (1989).
Kump, L. R. Interpreting carbon-isotope excursions; Strangelove oceans. Geology 19, 299–302 (1991).
D’Hondt, S., Donaghay, P., Zachos, J. C., Luttenberg, D. & Lindinger, M. Organic carbon fluxes and ecological recovery from the Cretaceous–Tertiary mass extinction. Science 282, 276–279 (1998).
Bown, P. R. Selective calcareous nannoplankton survivorship at the Cretaceous–Tertiary boundary. Geology 33, 653–656 (2005).
Hallock, P. Fluctuations in the trophic resource continuum: A factor in global diversity cycles? Paleoceanography 2, 457–471 (1987).
Johnson, K. R. & Hickey, L. J. in Global Catastrophes in Earth History (eds Sharpton, V. L. & Ward, P. D.) 433–444 (Geol. Soc. Am., 1990).
Schultz, P. H. & D’Hondt, S. L. Cretaceous–Tertiary (Chicxulub) impact angle and its consequences. Geology 24, 963–967 (1996).
Alvarez, W., Claeys, P. & Kieffer, S. W. Emplacement of Cretaceous–Tertiary boundary shocked quartz from Chicxulub crater. Science 269, 930–935 (1995).
Morgan, J. et al. Analyses of shocked quartz at the global K–P boundary indicate an origin from a single, high-angle, oblique impact at Chicxulub. Earth Planet. Sci. Lett. 251, 264–279 (2006).
Pope, K. O. Impact dust not the cause of the Cretaceous–Tertiary mass extinction. Geology 30, 99–102 (2002).
Covey, C., Ghan, S. J., Walton, J. J. & Weissman, P. R. in Global Catastrophes in Earth History (eds Sharpton, V. L. & Ward, P. D.) 263–270 (Geol. Soc. Am., 1990).
Anning, T., Harris, G. & Geider, R. J. Thermal acclimation in the marine diatom Chaetoceros calcitrans (Bacillariophyceae). Eur. J. Phycol. 36, 233–241 (2001).
Sandgren, C. D. Morphological variability in populations of chrysophycean resting cysts. 1. Genetic (interclonal) and encystment temperature effects on morphology. J. Phycol. 19, 64–70 (1983).
Cros, L., Kleijne, A., Zeltner, A., Billard, C. & Young, J. New examples of holococcolith–heterococcolith combination coccospheres and their implications for coccolithophorid biology. Mar. Micropaleontol. 39, 1–34 (2000).
Paasche, E. A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycologia 40, 503–529 (2002).
Lamolda, M. A., Melinte, M. C. & Kaiho, K. Nannofloral extinction and survivorship across the K/T boundary at Caravaca, southeastern Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 224, 27–52 (2005).
Antia, N. J. & Cheng, J. Y. The survival of axenic cultures of marine planktonic algae from prolonged exposure to darkness at 20 ∘C. Phycologia 9, 179–183 (1970).
Covey, C., Schneider, S. H. & Thompson, S. L. Global atmospheric effects of massive smoke injections from a nuclear war: Results from general circulation model simulations. Nature 308, 21–25 (1984).
Riebesell, U. et al. Reduced calcification of marine plankton in response to increased atmospheric CO2 . Nature 407, 362–367 (2000).
Iglesias-Rodriguez, M. D. et al. Phytoplankton calcification in a high-CO2 world. Science 320, 336–340 (2008).
Prinn, R. G. & Fegley, B. Jr Bolide impacts, acid rain, and biospheric traumas at the Cretaceous–Tertiary boundary. Earth Planet. Sci. Lett. 83, 1–15 (1987).
Toon, O. B. et al. in Geological Implications of Impacts of Large Asteroids and Comets on the Earth (eds Silver, L. T. & Schultz, P. H.) 187–200 (Geol. Soc. Am., 1982).
Bruland, K. W., Donat, J. R. & Hutchins, D. A. Interactive influences of bioactive trace-metals on biological production in oceanic waters. Limnol. Oceanogr. 36, 1555–1577 (1991).
Bruland, K. W., Knauer, G. A. & Martin, J. H. Cadmium in northeast Pacific waters. Limnol. Oceanogr. 23, 618–625 (1978).
Brand, L. E., Sunda, W. G. & Guillard, R. R. L. Limitation of marine phytoplankton reproductive rates by zinc, manganese, and iron. Limnol. Oceanogr. 28, 1182–1198 (1983).
Hilting, A. K., Kump, L. R. & Bralower, T. J. Variations in the oceanic vertical carbon isotope gradient and their implications for the Paleocene–Eocene biological pump. Paleoceanography 23, 1–15 (2008).
Bice, K. L. & Marotzke, J. Numerical evidence against reversed thermohaline circulation in the warm Paleocene/Eocene ocean. J. Geophys. Res. 106, 11529–11542 (2001).
Yeats, P. A. The distribution of trace metals in ocean waters. Sci. Total Environ. 72, 131–149 (1988).
Wunsch, C. & Heimbach, P. How long to oceanic tracer and proxy equilibrium? Quat. Sci. Rev. 27, 637–651 (2008).
Schueth, J. D., Jiang, S., Bralower, T. J. & Patzkowsky, M. E. A multivariate analysis of the recovery of calcareous nannoplankton and foraminifera from the Cretaceous–Paleogene mass extinction. GSA Abstr. Programs 40, 318-11 (2008).
Alagret, L. & Thomas, E. Cretaceous/Paleogene boundary bathyal paleo-environments in the central North Pacific (DSDP Site 465), the Northwestern Atlantic (ODP Site 1049), the Gulf of Mexico and the Tethys: The benthic foraminiferal record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 224, 53–82 (2005).
Keller, G. in Cretaceous-Tertiary Mass Extinctions: Biotic and Environmental Changes (eds MacLeod, N. & Keller, G.) 49–84 (W. W. Norton, 1996).
Acknowledgements
We thank J. Pospichal, P. Bown and H. Thierstein for sharing their unpublished nannofossil counts. This research was sponsored by the NASA Exobiology grant NNX07AK62G. Samples acquired from the Integrated Ocean Drilling Program funded by NSF.
Author information
Authors and Affiliations
Contributions
T.J.B., S.J. and M.E.P. designed the experiment, S.J., L.R.K. and J.D.S. conducted the analysis, T.J.B., L.R.K., M.E.P., S.J. and J.D.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1028 kb)
Rights and permissions
About this article
Cite this article
Jiang, S., Bralower, T., Patzkowsky, M. et al. Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary. Nature Geosci 3, 280–285 (2010). https://doi.org/10.1038/ngeo775
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo775
This article is cited by
-
Late Campanian-Maastrichtian in Pondicherry Area (Cauvery Basin) Southern India: Bioevents and Palaeoenvironmental Inferences from Planktonic Foraminifera
Journal of the Geological Society of India (2023)
-
Two-step extinction of Late Cretaceous marine vertebrates in northern Gulf of Mexico prolonged biodiversity loss prior to the Chicxulub impact
Scientific Reports (2020)
-
Rapid recovery of life at ground zero of the end-Cretaceous mass extinction
Nature (2018)
-
Palaeoecology: North–South recovery divide
Nature Ecology & Evolution (2017)
-
Rapid recovery of Patagonian plant–insect associations after the end-Cretaceous extinction
Nature Ecology & Evolution (2016)