Abstract
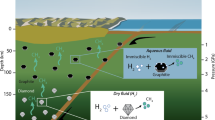
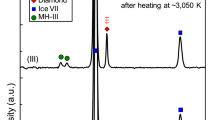
There is widespread evidence that petroleum originates from biological processes1,2,3. Whether hydrocarbons can also be produced from abiogenic precursor molecules under the high-pressure, high-temperature conditions characteristic of the upper mantle remains an open question. It has been proposed that hydrocarbons generated in the upper mantle could be transported through deep faults to shallower regions in the Earth’s crust, and contribute to petroleum reserves4,5. Here we use in situ Raman spectroscopy in laser-heated diamond anvil cells to monitor the chemical reactivity of methane and ethane under upper-mantle conditions. We show that when methane is exposed to pressures higher than 2 GPa, and to temperatures in the range of 1,000–1,500 K, it partially reacts to form saturated hydrocarbons containing 2–4 carbons (ethane, propane and butane) and molecular hydrogen and graphite. Conversely, exposure of ethane to similar conditions results in the production of methane, suggesting that the synthesis of saturated hydrocarbons is reversible. Our results support the suggestion that hydrocarbons heavier than methane can be produced by abiogenic processes in the upper mantle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tissot, B. P. & Welte, D. H. Petroleum Formation and Occurrence (Springer, 1984).
Whiticar, M. J., Faber, E. & Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs acetate fermentation—isotope evidence. Geochim. Cosmochim. Acta 50, 693–709 (1986).
Schoell, M. Multiple origins of methane in the earth. Chem. Geol. 71, 1–10 (1988).
Porfir’ev, V. B. Inorganic origin of petroleum. Am. Assoc. Petrol. Geol. Bull. 58, 3–33 (1974).
Krayushkin, V. A. Oil and gas fields of the abyssal genesis. D. I. Mendeleev J. All-Union. Chem. Soc. 31, 241–252 (1986).
Scott, H. P., Hemley, R. J. & Mao, H. et al. Generation of methane in the Earth’s mantle: In situ high pressure–temperature measurements of carbonate reduction. Proc. Natl Acad. Sci. USA. 101, 14023–14026 (2004).
Kenney, J. F., Kutcherov, V. G., Bendeliani, N. A. & Alekseev, V. A. The evolution of multicomponent systems at high pressures: VI. The thermodynamic stability of the hydrogen–carbon system: The genesis of hydrocarbons and the origin of petroleum. Proc. Natl Acad. Sci. USA. 99, 10976–10981 (2002).
Kutcherov, V. G., Bendeliani, N. A., Alekseev, V. A. & Kenney, J. F. Synthesis of hydrocarbons from minerals at pressures up to 5 GPa. Dokl. Akad. Nauk [in Russian] 387, 789–792 (2002).
Chen, J. Y., Jin, L. J., Dong, J. P., Zheng, H. F. & Liu, G. Y. Methane formation from CaCO3 reduction catalyzed by high pressure. Chin. Chem. Lett. 19, 475–478 (2008).
Nellis, W. J., Ree, F. H., Thiel, M. Van & Mitchell, A. C. Shock compression of liquid carbon monoxide and methane to 90 GPa (900 kbar). J. Chem. Phys. 75, 3055–3063 (1981).
Nellis, W. J., Hamilton, D. C. & Mitchell, A. C. Electrical conductivities of methane, benzene, and polybutene shock compressed to 60 GPa (600 kbar). J. Chem. Phys. 115, 1015–1019 (2001).
Ross, M. The ice layer in Uranus and Neptune—diamonds in the sky. Nature 292, 435–436 (1981).
Ancilotto, F., Chiarotti, G. L., Scandolo, S. & Tosatti, E. Dissociation of methane into hydrocarbons at extreme (planetary) pressure and temperature. Science 275, 1288–1290 (1997).
Kress, J. D., Bickham, S. R., Collins, L. A., Holian, B. L. & Goedecker, S. Tight-binding molecular dynamics of shock waves in methane. Phys. Rev. Lett. 83, 3896–3899 (1999).
Culler, T. S. & Schiferl, D. New chemical reactions in methane at high temperatures and pressures. J. Phys. Chem. 97, 703–706 (1993).
Benedetti, L. R. et al. Dissociation of CH4 at high pressures and temperatures: Diamond formation in giant planet interiors. Science 286, 100–102 (1999).
Zerr, A., Serghiou, G., Boehler, R. & Ross, M. Decomposition of alkanes at high pressure and temperatures. High Press. Res. 26, 23–32 (2006).
Hemley, R. J. & Mao, H. K. in Proc. 13th APS Conf. on Shock-compression of Condensed Matter (eds Furnish, M. D., Gupta, Y. M. & Forbes, J. W.) 17–26 (AIP, 2004).
Chen, J. Y., Jin, L. J., Dong, J. P. & Zheng, H. F. In situ Raman spectroscopy study on dissociation of methane at high temperatures and at high pressures. Chin. Phys. Lett. 25, 780–782 (2008).
Hirai, H., Konagai, K., Kawamura, T., Yamamoto, Y. & Yagi, T. Polymerization and diamond formation from melting methane and their implications in ice layer of giant planets. Phys. Earth Planet. Inter. 174, 242–246 (2009).
Somayazulu, M. S., Finger, L. W., Hemley, R. J. & Mao, H. K. High-pressure compounds in methane–hydrogen mixtures. Science 271, 1400–1402 (1996).
Huebner, J. S. in Research Techniques for High Pressure and High Temperature (ed. Ulmer, G. C.) 123–177 (Springer, 1972).
Sherwood Lollar, B. S. et al. Unravelling abiogenic and biogenic sources of methane in the Earth’s deep subsurface. Chem. Geol. 226, 328–339 (2006).
Goncharov, A. F. et al. Dynamic ionization of water under extreme conditions. Phys. Rev. Lett. 94, 125508 (2005).
Yagi, T. & Suzuki, H. Melting curve of methane to 4.8 GPa determined by the Ruby pressure–temperature marker. Proc. Japan Acad. Ser. B 66, 167–172 (1990).
Woodland, A. B. & Koch, M. Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal craton, Southern Africa. Earth. Planet. Sci. Lett. 214, 295–310 (2003).
McCammon, C. & Kopylova, M. G. A redox profile of the Slave mantle and oxygen fugacity control in the cratonic mantle. Contrib. Mineral. Petrol. 148, 55–68 (2004).
Simakov, S. K. Redox state of eclogites and peridotites from sub-cratonic upper mantle and a connection with diamond genesis. Contrib. Mineral. Petrol. 151, 282–296 (2006).
Pollack, H. N. & Chapman, D. S. On the regional variation of heat flow, geotherms, and lithospheric thickness. Tectonophysics 38, 279–296 (1977).
Acknowledgements
We thank K. Litasov, Y. Fei, J. C. Crowhurst, M. Somayazulu, V. Struzhkin, R. Cohen, D. Foustoukos, J. Montoya, T. Strobel and R. J. Hemley for valuable information, comments and discussions. We thank S. Sinogeikin for help with X-ray diffraction experiments. A.K. acknowledges the support from INTAS through YSF Ref. No. 06-1000014-6546. V.G.K. acknowledges the support from INTAS Ref. No. 06-1000013-8750. We acknowledge support by the US Department of Energy (DOE)/National Nuclear Security Agency through the Carnegie/DOE Alliance Center, NSF- EAR, the W. M. Keck Foundation and the Carnegie Institution of Washington. Use of the HPCAT facility (Carnegie Institution of Washington) was supported by DOE-BES, DOE-NNSA (CDAC), NSF, DOD–TACOM and the W. M. Keck Foundation. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38.
Author information
Authors and Affiliations
Contributions
V.G.K designed the study. A.F.G. and A.K. designed the experiments. A.K. and A.F.G. carried out the experiments and reduced the data. A.K. carried out the data analysis. A.F.G. wrote the manuscript with substantial contributions made by the other authors. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 627 kb)
Rights and permissions
About this article
Cite this article
Kolesnikov, A., Kutcherov, V. & Goncharov, A. Methane-derived hydrocarbons produced under upper-mantle conditions. Nature Geosci 2, 566–570 (2009). https://doi.org/10.1038/ngeo591
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo591
This article is cited by
-
Diamond precipitation dynamics from hydrocarbons at icy planet interior conditions
Nature Astronomy (2024)
-
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies
Progress in Earth and Planetary Science (2023)
-
In-situ abiogenic methane synthesis from diamond and graphite under geologically relevant conditions
Nature Communications (2021)
-
Raman Spectroscopy Study on Chemical Transformations of Propane at High Temperatures and High Pressures
Scientific Reports (2020)
-
Formation of complex hydrocarbon systems from methane at the upper mantle thermobaric conditions
Scientific Reports (2020)