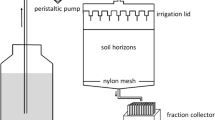
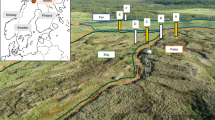
Abstract
Colloids, such as submicrometre mineral particles or bacterial cells, can act as carriers enhancing the mobility of poorly soluble contaminants in subsurface environments1,2. In sulphate-reducing soils and sediments, metal sulphide precipitation has been proposed3,4,5,6 to generate contaminant-bearing sulphide colloids, which could transport contaminants traditionally thought to be immobilized by metal sulphide formation7. However, direct evidence for such a process is lacking. Here, we report the composition and morphology of pore-water colloids formed in contaminated floodplain soil when flooded with synthetic river water over a four-week period. We show that, on flooding, bacteria dispersed in the pore water mobilize copper by inducing biomineralization of metallic copper(0). We suggest that copper(0) crystals form by disproportionation of copper(I), which is released by copper-stressed bacteria to maintain copper homeostasis8,9. Sulphate reduction, which started on the fourth day of flooding, resulted in the mobilization of cadmium and lead, which were partitioned to copper-rich sulphide colloids showing two types of morphology: bacterium-associated ∼50–150-nm-diameter hollow particles formed through copper(0) transformation, and dispersed <50 nm nanoparticles, probably formed through homogeneous precipitation. The slow deposition of both types of sulphide colloid ensured elevated contaminant concentrations in the pore water for weeks. Our findings imply that colloid formation can enhance contaminant release from periodically sulphate-reducing soils and sediments, potentially polluting surface- and groundwaters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McCarthy, J. F. & Zachara, J. M. Subsurface transport of contaminants. Environ. Sci. Tech. 23, 496–502 (1989).
Kretzschmar, R., Borkovec, M., Grolimund, D. & Elimelech, M. Mobile subsurface colloids and their role in contaminant transport. Adv. Agron. 66, 121–193 (1999).
Horzempa, L. M. & Helz, G. R. Controls on the stability of sulfide soils: Colloidal covellite as an example. Geochim. Cosmochim. Acta 43, 1645–1650 (1979).
Gammons, C. H. & Frandsen, A. K. Fate and transport of metals in H2S-rich waters at a treatment wetland. Geochem. Trans. 2, 1–15 (2001).
Moreau, J. W., Webb, R. I. & Banfield, J. F. Ultrastructure, aggregation-state, and crystal growth of biogenic nanocrystalline sphalerite and wurtzite. Am. Mineral. 89, 950–960 (2004).
Lau, B. L. T. & Hsu-Kim, H. Precipitation and growth of zinc sulfide nanoparticles in the presence of thiol-containing natural organic ligands. Environ. Sci. Tech. 42, 7236–7241 (2008).
Kirk, G. The Biogeochemistry of Submerged Soils (Wiley, 2004).
Rensing, C. & Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27, 197–213 (2003).
Outten, F. W., Huffman, D. L., Hale, J. A. & O’Halloran, T. V. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276, 30670–30677 (2001).
Hochella, M. F. Jr. et al. Direct observation of heavy metal–mineral association from the Clark Fork River Superfund Complex: Implications for metal transport and bioavailability. Geochim. Cosmochim. Acta 69, 1651–1663 (2005).
Voegelin, A., Weber, F.-A. & Kretzschmar, R. Distribution and speciation of arsenic around roots in a contaminated riparian floodplain soil: Micro-XRF element mapping and EXAFS spectroscopy. Geochim. Cosmochim. Acta 71, 5804–5820 (2007).
Luther, G. W. III & Rickard, D. T. Metal sulfide cluster complexes and their biogeochemical importance in the environment. J. Nanopart. Res. 7, 389–407 (2005).
Rozan, T. F., Lassman, M. E., Ridge, D. P. & Luther, G. W. III. Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic rivers. Nature 406, 879–882 (2000).
Luther, G. W. III. et al. Aqueous copper sulfide clusters as intermediates during copper sulfide formation. Environ. Sci. Tech. 36, 394–402 (2002).
Ferris, F. G., Fyfe, W. S. & Beveridge, T. J. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 63, 225–232 (1987).
Bebie, J., Schoonen, M. A. A., Fuhrmann, M. & Strongin, D. R. Surface charge development on transition metal sulfides: An electrokinetic study. Geochim. Cosmochim. Acta 62, 633–642 (1998).
Tufenkji, N. Modeling microbial transport in porous media: Traditional approaches and recent developments. Adv. Water Resour. 30, 1455–1469 (2007).
Gustafsson, Ö. & Gschwend, P. M. Aquatic colloids: Concepts, definitions, and current challenges. Limnol. Oceanogr. 42, 519–528 (1997).
Gschwend, P. M. & Reynolds, M. D. Monodisperse ferrous phosphate colloids in an anoxic groundwater plume. J. Contam. Hydrol. 1, 309–327 (1987).
Jacobs, L., Emerson, S. & Skei, J. Partitioning and transport of metals across the O2/H2S interface in a permanently anoxic basin: Framvaren Fjord, Norway. Geochim. Cosmochim. Acta 49, 1433–1444 (1985).
Lowry, G. V., Shaw, S., Kim, C. S., Rytuba, J. J. & Brown, G. E. Jr. Macroscopic and microscopic observations of particle-facilitated mercury transport from New Idria and Sulphur Bank mercury mine tailings. Environ. Sci. Tech. 38, 5101–5111 (2004).
Kau, L.-S., Spira-Solomon, D. J., Penner-Hahn, J. E., Hodgson, K. O. & Solomon, E. I. X-ray absorption edge determination of the oxidation state and coordination number of copper: Application to the type 3 site in Rhus vernicifera laccase and its reaction with oxygen. J. Am. Chem. Soc. 109, 6433–6442 (1987).
Peariso, K., Huffman, D. L., Penner-Hahn, J. E. & O’Halloran, T. V. The PcoC copper resistance protein coordinates Cu(I) via novel S-methionine interactions. J. Am. Chem. Soc. 125, 342–343 (2003).
Davis, A. V. & O’Halloran, T. V. A place for thioether chemistry in cellular copper ion recognition and trafficking. Nature Chem. Biol. 4, 148–151 (2008).
Manceau, A. et al. Formation of metallic copper nanoparticles at the soil–root interface. Environ. Sci. Tech. 42, 1766–1772 (2008).
Reith, F., Lengke, M. F., Falconer, D., Craw, D. & Southam, G. The geomicrobiology of gold. ISME J. 1, 567–584 (2007).
Pattrick, R. A. D. et al. The structure of amorphous copper sulfide precipitates: An X-ray absorption study. Geochim. Cosmochim. Acta 61, 2023–2036 (1997).
Yin, Y. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304, 711–714 (2004).
Moreau, J. W. et al. Extracellular proteins limit the dispersal of biogenic nanoparticles. Science 316, 1600–1603 (2007).
Karlsson, T., Persson, P. & Skyllberg, U. Complexation of copper(II) in organic soils and in dissolved organic matter—EXAFS evidence for chelate ring structures. Environ. Sci. Tech. 40, 2623–2628 (2006).
Acknowledgements
We thank K. Barmettler for support in the laboratory, S. Mangold for assistance at the XAS beamline at ANKA, and U. Skyllberg and J. F. W. Mosselmans for sharing reference XAS spectra. We thank ANKA Angströmquelle Karlsruhe GmbH, Germany, for allocation of synchrotron beamtime and acknowledge the support of the Electron Microscopy Centre (EMEZ) of ETH Zurich, Switzerland. Funding from the Swiss State Secretariat for Education and Research (contract 03.0353-1) as a contribution to the EU project AquaTerra is acknowledged.
Author information
Authors and Affiliations
Contributions
F.-A.W. designed and performed all experiments and measurements, evaluated the results and wrote the manuscript as part of his PhD thesis. A.V. conducted EXAFS analyses and contributed to writing. R.Ka. conducted TEM analyses. A.V. and R.Kr. initiated and supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Table S1
Supplementary Information (PDF 794 kb)
Rights and permissions
About this article
Cite this article
Weber, FA., Voegelin, A., Kaegi, R. et al. Contaminant mobilization by metallic copper and metal sulphide colloids in flooded soil. Nature Geosci 2, 267–271 (2009). https://doi.org/10.1038/ngeo476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo476
This article is cited by
-
A critical review on the biological impact of natural organic matter on nanomaterials in the aquatic environment
Carbon Research (2022)
-
Biogeochemical Control on the Mobilization of Cd in Soil
Current Pollution Reports (2021)
-
Potential Effects of Episodic Deposition on Nutrients and Heavy Metals in Decomposing Litters of Suaeda glauca in Salt Marsh of the Yellow River Estuary, China
Chinese Geographical Science (2020)
-
Fate and risk of metal sulfide nanoparticles in the environment
Environmental Chemistry Letters (2020)
-
Effects of ultrasonic stimulation on the transport of different-sized particles in porous media
Hydrogeology Journal (2020)