Abstract
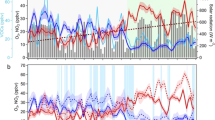
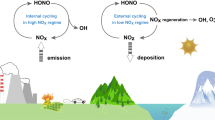
The influence of nitrogen oxides (NOx) on daytime atmospheric oxidation cycles is well known, with clearly defined high- and low-NOx regimes. During the day, oxidation reactions—which contribute to the formation of secondary pollutants such as ozone—are proportional to NOx at low levels, and inversely proportional to NOx at high levels. Night-time oxidation of volatile organic compounds also influences secondary pollutants but lacks a similar clear definition of high- and low-NOx regimes, even though such regimes exist. Decreases in anthropogenic NOx emissions in the US and Europe coincided with increases in Asia over the last 10 to 20 years, and have altered both daytime and nocturnal oxidation cycles. Here we present measurements of chemical species in the lower atmosphere from day- and night-time research flights over the southeast US in 1999 and 2013, supplemented by atmospheric chemistry simulations. We find that night-time oxidation of biogenic volatile organic compounds (BVOC) is NOx-limited when the ratio of NOx to BVOC is below approximately 0.5, and becomes independent of NOx at higher ratios. The night-time ratio of NOx to BVOC in 2013 averaged 0.6 aloft. We suggest that night-time oxidation in the southeast US is in transition between NOx-dominated and ozone-dominated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fuentes, J. D. et al. Biogenic hydrocarbons in the atmospheric boundary layer: a review. Bull. Am. Meteorol. Soc. 81, 1537–1575 (2000).
Atkinson, R. & Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 103, 4605–4638 (2003).
Atkinson, R. & Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos. Environ. 37, S197–S219 (2003).
Draper, D. C., Farmer, D. K., Desyaterik, Y. & Fry, J. L. A qualitative comparison of secondary organic aerosol yields and composition from ozonolysis of monoterpenes at varying concentrations of NO2 . Atmos. Chem. Phys. 15, 12267–12281 (2015).
Brown, S. S. & Stutz, J. Nighttime radical observations and chemistry. Chem. Soc. Rev. 41, 6405–6447 (2012).
Stull, R. B. An Introduction to Boundary Layer Meteorology (Kluwer Academic, 1988).
Brown, S. S. et al. The effects of NOx control and plume mixing on nighttime chemical processing of plumes from coal-fired power plants. J. Geophys. Res. 117, D07304 (2012).
Boyd, C. M. et al. Secondary organic aerosol formation from the β-pinene + NO3 system: effect of humidity and peroxy radical fate. Atmos. Chem. Phys. 15, 7497–7522 (2015).
Chung, S. H. & Seinfeld, J. H. Global distribution and climate forcing of carbonaceous aerosols. J. Geophys. Res. 107, 4407 (2002).
Fry, J. L. et al. Secondary organic aerosol formation and organic nitrate yield from NO3 oxidation of biogenic hydrocarbons. Environ. Sci. Technol. 48, 11944–11953 (2014).
Fry, J. L. et al. SOA from limonene: role of NO3 in its generation and degradation. Atmos. Chem. Phys. 11, 3879–3894 (2011).
Fry, J. L. et al. Organic nitrate and secondary organic aerosol yield from NO3 oxidation of β-pinene evaluated using a gas-phase kinetics/aerosol partitioning model. Atmos. Chem. Phys. 9, 1431–1449 (2009).
Fry, J. L. & Sackinger, K. Model evaluation of NO3 secondary organic aerosol (SOA) source and heterogeneous organic aerosol (OA) sink in the Western United States. Atmos. Chem. Phys. 12, 8797–8811 (2012).
Griffin, R. J., Cocker, D. R. III, Flagan, R. C. & Seinfeld, J. H. Organic aerosol formation from the oxidation of biogenic hydrocarbons. J. Geophys. Res. 104, 3555–3567 (1999).
Hoyle, C. R., Berntsen, T., Myhre, G. & Isaksen, I. S. A. Secondary organic aerosol in the global aerosol—chemical transport model Oslo CTM2. Atmos. Chem. Phys. 7, 5675–5694 (2007).
Ng, N. L. et al. Secondary organic aerosol (SOA) formation from reaction of isoprene with nitrate radicals (NO3). Atmos. Chem. Phys. 8, 4117–4140 (2008).
Pye, H. O. T., Chan, A. W. H., Barkley, M. P. & Seinfeld, J. H. Global modeling of organic aerosol: the importance of reactive nitrogen (NOx and NO3). Atmos. Chem. Phys. 10, 11261–11276 (2010).
Pye, H. O. T. et al. Modeling the current and future roles of particulate organic nitrates in the southeastern United States. Environ. Sci. Technol. 49, 14195–14203 (2015).
Rollins, A. W. et al. Isoprene oxidation by nitrate radical: alkyl nitrate and secondary organic aerosol yields. Atmos. Chem. Phys. 9, 6685–6703 (2009).
Zheng, Y. et al. Limited effect of anthropogenic nitrogen oxides on secondary organic aerosol formation. Atmos. Chem. Phys. 15, 13487–13506 (2015).
Hallquist, M., Wängberg, I., Ljungstrom, E., Barnes, I. & Becker, K. H. Aerosol and product yields from NO3 radical-initiated oxidation of selected monoterpenes. Environ. Sci. Technol. 33, 553–559 (1999).
Lee, B. H. et al. Highly functionalized organic nitrates in the southeast United States: contribution to secondary organic aerosol and reactive nitrogen budgets. Proc. Natl Acad. Sci. USA 113, 1516–1521 (2016).
Perring, A. E. et al. A product study of the isoprene+NO3 reaction. Atmos. Chem. Phys. 9, 4945–4946 (2009).
Fisher, J. A. et al. Organic nitrate chemistry and its implications for nitrogen budgets in an isoprene- and monoterpene-rich atmosphere: constraints from aircraft (SEAC4RS) and ground-based (SOAS) observations in the Southeast US. Atmos. Chem. Phys. 16, 5969–5991 (2016).
Horowitz, L. W. et al. Observational constraints on the chemistry of isoprene nitrates over the eastern United States. J. Geophys. Res. 112, D12S08 (2007).
von Kuhlmann, R., Lawrence, M. G., Pöschl, U. & Crutzen, P. J. Sensitivities in global scale modeling of isoprene. Atmos. Chem. Phys. 4, 1–17 (2004).
Mao, J. et al. Ozone and organic nitrates over the eastern United States: sensitivity to isoprene chemistry. J. Geophys. Res. 118, 2013JD020231 (2013).
Zaveri, R. A. et al. Nighttime chemical evolution of aerosol and trace gases in a power plant plume: implications for secondary organic nitrate and organosulfate aerosol formation, NO3 radical chemistry, and N2O5 heterogeneous hydrolysis. J. Geophys. Res. 115, D12304 (2010).
Zaveri, R. A. et al. Overnight atmospheric transport and chemical processing of photochemically aged Houston urban and petrochemical industrial plume. J. Geophys. Res. 115, D23303 (2010).
Stone, D. et al. Radical chemistry at night: comparisons between observed and modelled HOx, NO3 and N2O5 during the RONOCO project. Atmos. Chem. Phys. 14, 1299–1321 (2014).
Brown, S. S. et al. Nocturnal isoprene oxidation over the Northeast United States in summer and its impact on reactive nitrogen partitioning and secondary organic aerosol. Atmos. Chem. Phys. 9, 3027–3042 (2009).
Brown, S. S. et al. Vertical profiles in NO3 and N2O5 measured from an aircraft: Results from the NOAA P-3 and surface platforms during NEAQS 2004. J. Geophys. Res. 112, D22304 (2007).
Brown, S. S. et al. Biogenic VOC oxidation and organic aerosol formation in an urban nocturnal boundary layer: aircraft vertical profiles in Houston, TX. Atmos. Chem. Phys. 13, 11317–11337 (2013).
Goldstein, A. H., Koven, C. D., Heald, C. L. & Fung, I. Y. Biogenic carbon and anthropogenic pollutants combine to form a cooling haze over the southeastern United States. Proc. Natl Acad. Sci. USA 106, 8835–8840 (2009).
Guenther, A. et al. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 6, 3181–3210 (2006).
Warneke, C. et al. Instrumentation and measurement strategy for the NOAA SENEX aircraft campaign as part of the Southeast Atmosphere Study 2013. Atmos. Meas. Tech. 9, 3063–3093 (2016).
Brown, S. S. et al. Budgets for nocturnal VOC oxidation by nitrate radicals aloft during the 2006 Texas Air Quality Study. J. Geophys. Res. 116, D24305 (2011).
Emmerson, K. M. & Evans, M. J. Comparison of tropospheric gas-phase chemistry schemes for use within global models. Atmos. Chem. Phys. 9, 1831–1845 (2009).
Jenkin, M. E., Young, J. C. & Rickard, A. R. The MCM v3.3.1 degradation scheme for isoprene. Atmos. Chem. Phys. 15, 11433–11459 (2015).
Kleinman, L. I. The dependence of tropospheric ozone production rate on ozone precursors. Atmos. Environ. 39, 575–586 (2005).
Russell, A. R., Valin, L. C. & Cohen, R. C. Trends in OMI NO2 observations over the United States: effects of emission control technology and the economic recession. Atmos. Chem. Phys. 12, 12197–12209 (2012).
Millet, D. B. et al. Nighttime chemistry and morning isoprene can drive urban ozone downwind of a major deciduous forest. Environ. Sci. Technol. 50, 4335–4342 (2016).
Attwood, A. R. et al. Trends in sulfate and organic aerosol mass in the Southeast US: impact on aerosol optical depth and radiative forcing. Geophys. Res. Lett. 41, 7701–7709 (2014).
Kiendler-Scharr, A. et al. Ubiquity of organic nitrates from nighttime chemistry in the European submicron aerosol. Geophys. Res. Lett. 43, 7735–7744 (2016).
Rollins, A. W. et al. Evidence for NOx control over nighttime SOA formation. Science 337, 1210–1212 (2012).
Surratt, J. D. et al. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl Acad. Sci. USA 107, 6640–6645 (2010).
Xu, L. et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proc. Natl Acad. Sci. USA 112, 37–42 (2015).
Pollack, I. B., Lerner, B. M. & Ryerson, T. B. Evaluation of ultraviolet light-emitting diodes for detection of atmospheric NO2 by photolysis—chemiluminescence. J. Atmos. Chem. 65, 111–125 (2010).
Ryerson, T. B. et al. Design and initial characterization of an inlet for gas-phase NOy measurements from aircraft. J. Geophys. Res. 104, 5483–5492 (1999).
Dubé, W. P. et al. Aircraft instrument for simultaneous in-situ measurements of NO3 and N2O5 via cavity ring-down spectroscopy. Rev. Sci. Instr. 77, 034101 (2006).
Wagner, N. L. et al. Diode laser-based cavity ring-down instrument for NO3, N2O5, NO, NO2 and O3 from aircraft. Atmos. Meas. Tech. 4, 1227–1240 (2011).
de Gouw, J. A. & Warneke, C. Measurements of volatile organic compounds in the Earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass. Spec. Rev. 26, 223–257 (2007).
Lerner, B. M. et al. An improved, automated whole air sampler and gas chromatography mass spectrometry analysis system for volatile organic compounds in the atmosphere. Atmos. Meas. Tech. 10, 291–313 (2017).
Cazorla, M. et al. A new airborne laser-induced fluorescence instrument for in situ detection of formaldehyde throughout the troposphere and lower stratosphere. Atmos. Meas. Tech. 8, 541–552 (2015).
Acknowledgements
F.N.K. and J.K. acknowledge the US EPA Science to Achieve Results (STAR) program grant 83540601. This research has not been subjected to any EPA review and therefore does not necessarily reflect the views of the agency, and no official endorsement should be inferred. J.K. also acknowledges support from NASA Headquarters under the NASA Earth and Space Science Fellowship Program—grant NNX14AK97H. J.L.F. gratefully acknowledges funding from the NOAA Climate Program Office’s AC4 program (Grant No. NA13OAR4310070).
Author information
Authors and Affiliations
Contributions
The analysis presented in this paper was performed by P.M.E. and S.S.B. Model simulations were performed by P.M.E. All other co-authors were instrumental in the collection of the data used in this analysis. The manuscript was written by P.M.E. and S.S.B. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 9064 kb)
Rights and permissions
About this article
Cite this article
Edwards, P., Aikin, K., Dube, W. et al. Transition from high- to low-NOx control of night-time oxidation in the southeastern US. Nature Geosci 10, 490–495 (2017). https://doi.org/10.1038/ngeo2976
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2976
This article is cited by
-
Recent Advancement in Organic Aerosol Understanding: a Review of Their Sources, Formation, and Health Impacts
Water, Air, & Soil Pollution (2023)
-
Suppression of anthropogenic secondary organic aerosol formation by isoprene
npj Climate and Atmospheric Science (2022)
-
Anthropogenic Effects on Biogenic Secondary Organic Aerosol Formation
Advances in Atmospheric Sciences (2021)