Abstract
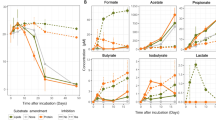
Permeable sediments are common across continental shelves and are critical contributors to marine biogeochemical cycling. Organic matter in permeable sediments is dominated by microalgae, which as eukaryotes have different anaerobic metabolic pathways to bacteria and archaea. Here we present analyses of flow-through reactor experiments showing that dissolved inorganic carbon is produced predominantly as a result of anaerobic eukaryotic metabolic activity. In our experiments, anaerobic production of dissolved inorganic carbon was consistently accompanied by large dissolved H2 production rates, suggesting the presence of fermentation. The production of both dissolved inorganic carbon and H2 persisted following administration of broad spectrum bactericidal antibiotics, but ceased following treatment with metronidazole. Metronidazole inhibits the ferredoxin/hydrogenase pathway of fermentative eukaryotic H2 production, suggesting that pathway as the source of H2 and dissolved inorganic carbon production. Metabolomic analysis showed large increases in lipid production at the onset of anoxia, consistent with documented pathways of anoxic dark fermentation in microalgae. Cell counts revealed a predominance of microalgae in the sediments. H2 production was observed in dark anoxic cultures of diatoms (Fragilariopsis sp.) and a chlorophyte (Pyramimonas) isolated from the study site, substantiating the hypothesis that microalgae undertake fermentation. We conclude that microalgal dark fermentation could be an important energy-conserving pathway in permeable sediments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 December 2016
In the version of this Article originally published, the middle bar in Fig. 1b was mislabelled and should have been labelled NO2-. This has been corrected in all versions of the Article.
References
MacIntyre, H. L., Geider, R. J. & Miller, D. C. Microphytobenthos: the ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. 1. Distribution, abundance and primary production. Estuaries 19, 186–201 (1996).
Jahnke, R. A., Nelson, J. R., Marinelli, R. L. & Eckman, J. E. Benthic flux of biogenic elements on the southeastern US continental shelf: influence of pore water advective transport and benthic microalgae. Cont. Shelf Res. 20, 109–127 (2000).
de Jonge, V. N. Fluctuations in the organic carbon to chlorophyll a ratios for estuarine benthic diatom populations. Mar. Ecol. Progr. Ser. 2, 345–353 (1980).
Light, B. B. J. Distribution and Primary Productivity of Microphytobenthos in Port Phillip Bay 67 (CSIRO Institute of Natural Resources and Environment, 1998).
Rusch, A., Forster, S. & Huettel, M. Bacteria, diatoms and detritus in an intertidal sandflat subject to advective transport across the water-sediment interface. Biogeochemistry 55, 1–27 (2001).
Evrard, V., Cook, P. L. M., Veuger, B., Huettel, M. & Middelburg, J. J. Tracing incorporation and pathways of carbon and nitrogen in microbial communities of photic subtidal sands. Aquat. Microb. Ecol. 53, 257–269 (2008).
Valdemarsen, T. & Kristensen, E. Degradation of dissolved organic monomers and short-chain fatty acids in sandy marine sediment by fermentation and sulfate reduction. Geochim. Cosmochim. Acta 74, 1593–1605 (2010).
Boudreau, B. P. et al. Permeable marine sediments: overturning an old paradigm. Eos 82, 133–136 (2001).
Veuger, B. & van Oevelen, D. Long-term pigment dynamics and diatom survival in dark sediment. Limnol. Oceanogr. 56, 1065–1074 (2011).
Kamp, A., de Beer, D., Nitsch, J. L., Lavik, G. & Stief, P. Diatoms respire nitrate to survive dark and anoxic conditions. Proc. Natl Acad. Sci. USA 108, 5649–5654 (2011).
Zhang, L. P., Happe, T. & Melis, A. Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga). Planta 214, 552–561 (2002).
Atteia, A., van Lis, R., Tielens, A. G. M. & Martin, W. F. Anaerobic energy metabolism in unicellular photosynthetic eukaryotes. Biochim. Biophys. Acta 1827, 210–223 (2013).
Evrard, V., Glud, R. N. & Cook, P. L. M. The kinetics of denitrification in permeable sediments. Biogeochemistry 113, 563–572 (2013).
Marchant, H. K. et al. Coupled nitrification-denitrification leads to extensive N loss in subtidal permeable sediments. Limnol. Oceanogr. 61, 1033–1048 (2016).
Pallud, C., Meile, C., Laverman, A. M., Abell, J. & Van Cappellen, P. The use of flow-through sediment reactors in biogeochemical kinetics: methodology and examples of applications. Mar. Chem. 106, 256–271 (2007).
Cook, P. L. M., Wenzhofer, F., Glud, R. N., Janssen, F. & Huettel, M. Benthic solute exchange and carbon mineralization in two shallow subtidal sandy sediments: effect of advective pore-water exchange. Limnol. Oceanogr. 52, 1943–1963 (2007).
Stief, P., Kamp, A. & de Beer, D. Role of diatoms in the spatial-temporal distribution of intracellular nitrate in intertidal sediment. PLoS ONE 8, e73257 (2013).
Lutzhoft, H. C. H., Halling-Sorensen, B. & Jorgensen, S. E. Algal toxicity of antibacterial agents applied in Danish fish farming. Arch. Environ. Contam. Toxicol. 36, 1–6 (1999).
Hoehler, T. M., Albert, D. B., Alperin, M. J. & Martens, C. S. Acetogenesis from CO2 in an anoxic marine sediment. Limnol. Oceanogr. 44, 662–667 (1999).
Finke, N. & Jorgensen, B. B. Response of fermentation and sulfate reduction to experimental temperature changes in temperate and Arctic marine sediments. ISME J. 2, 815–829 (2008).
Halling-Sorensen, B., Lutzhoft, H. C. H., Andersen, H. R. & Ingerslev, F. Environmental risk assessment of antibiotics: comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 46, 53–58 (2000).
Robinson, A. A., Belden, J. B. & Lydy, M. J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 24, 423–430 (2005).
Kummerer, K., Al-Ahmad, A. & Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 40, 701–710 (2000).
Martins, N. et al. Ecotoxicological effects of ciprofloxacin on freshwater species: data integration and derivation of toxicity thresholds for risk assessment. Ecotoxicology 21, 1167–1176 (2012).
Catalanotti, C., Yang, W., Posewitz, M. C. & Grossman, A. R. Fermentation metabolism and its evolution in algae. Front. Plant. Sci. 4, 150 (2013).
Greening, C. et al. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 10, 761–777 (2016).
Dubini, A., Mus, F., Seibert, M., Grossman, A. R. & Posewitz, M. C. Flexibility in anaerobic metabolism as revealed in a mutant of Chlamydomonas reinhardtii lacking hydrogenase activity. J. Biol. Chem. 284, 7201–7213 (2009).
Lloyd, D. & Kristensen, B. Metronidazole inhibition of hydrogen production in vivo in drug sensitive and resistant strains of Trichomonas vaginalis. J. Gen. Microbiol. 131, 849–853 (1985).
Ohta, S., Miyamoto, K. & Miura, Y. Hydrogen evolution as a consumption mode of reducing equivalents in green algal fermentation. Plant Physiol. 83, 1022–1026 (1987).
Mus, F., Dubini, A., Seibert, M., Posewitz, M. C. & Grossman, A. R. Anaerobic acclimation in Chlamydomonas reinhardtii—anoxic gene expression, hydrogenase induction, and metabolic pathways. J. Biol. Chem. 282, 25475–25486 (2007).
Inui, H., Miyatake, K., Nakano, Y. & Kitaoka, S. Wax ester fermentation in Euglena gracilis. FEBS Lett. 150, 89–93 (1982).
Tucci, S., Vacula, R., Krajcovic, J., Proksch, P. & Martin, W. Variability of wax ester fermentation in natural and bleached Euglena gracilis strains in response to oxygen and the elongase inhibitor flufenacet. J. Eukaryot. Microbiol. 57, 63–69 (2010).
Schwartz, E., Fritsch, J. & Friedrich, B. in The Prokaryotes: Prokaryotic Physiology and Biochemistry (eds Rosenberg, E. et al.) 119–199 (Springer, 2013).
Hirst, A. D. & Goldberg, D. M. Application of new ultra-micro spectrophotometric determination for serum hydroxybutyrate dehydrogenase activity to diagnosis of myocardial infarction. Br. Heart J. 32, 114–121 (1970).
Dyksma, S. et al. Ubiquitous Gammaproteobacteria dominate dark carbon fixation in coastal sediments. ISME J. 10, 1939–1953 (2016).
Melis, A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226, 1075–1086 (2007).
Dupreez, D. R. & Bate, G. C. Dark survival of the surf diatom Anaulus australis Drebes et Schulz. Bot. Mar. 35, 315–319 (1992).
Heisterkamp, I. M., Kamp, A., Schramm, A. T., de Beer, D. & Stief, P. Indirect control of the intracellular nitrate pool of intertidal sediment by the polychaete Hediste diversicolor. Mar. Ecol. Prog. Ser. 445, 181–192 (2012).
Canfield, D. E. et al. Pathways of organic carbon oxidation in 3 continental margin sediments. Mar. Geol. 113, 27–40 (1993).
Canfield, D. E., Thamdrup, B. & Hansen, J. W. The anaerobic degredation of organic matter in Danish coastal sediments—iron reduction, manganese reduction and sulfate reduction. Geochim. Cosmochim. Acta 57, 3867–3883 (1993).
Gattuso, J. P. et al. Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences 3, 489–513 (2006).
Evrard, V., Glud, R. N. & Cook, P. L. M. The kinetics of denitrification in permeable sediments. Biogeochemistry 113, 563–572 (2012).
Bourke, M., Kessler, A. & Cook, P. Influence of buried Ulva lactuca on denitrification in permeable sediments. Mar. Ecol. Prog. Ser. 498, 85–94 (2014).
Nielsen, L. P. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol. Ecol. 86, 357–362 (1992).
Oshima, M. et al. Highly sensitive determination method for total carbonate in water samples by flow injection analysis coupled with gas-diffusion separation. Anal. Sci. 17, 1285–1290 (2001).
Stookey, L. L. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42, 779–781 (1970).
Viollier, E., Inglett, P. W., Hunter, K., Roychoudhury, A. N. & Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 15, 785–790 (2000).
Fonselius, S. H. in Methods of Seawater Analysis (ed. Grasshoff, K.) 91–100 (Springer, 2007).
Anderson, L. G., Turner, D. R., Wedborg, M. & Dyrssen, D. in Methods of Seawater Analysis (eds Grasshoff, K., Kremling, K. & Ehrhardt, M.) 127–148 (Springer, 2007).
Jeffrey, S. W. & Humphrey, G. F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pfl. 167, 191–194 (1975).
Godzien, J. et al. In-vial dual extraction liquid chromatography coupled to mass spectrometry applied to streptozotocin-treated diabetic rats. Tips and pitfalls of the method. J. Chromatogr. A 1304, 52–60 (2013).
Kind, T. et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 81, 10038–10048 (2009).
Hasle, G. R. & Syvertsen, E. E. in Identifying Marine Diatoms and Dinoflagellates (eds Tomas, C. R, Hasle, G. R., Syvertsen, E. E., Steidinger, K. A. & Tangen, K.) 5–385 (Elsevier, 1997).
Acknowledgements
This work was supported by the Australian Research Council grant DP1096457 awarded to P.L.M.C. and R.N.G. R.N.G. was additionally supported by Danish Council for Independent Research, Natural Sciences, FNU, (0602-02276B) and the European Research Council through an Advanced Grant (ERC-2010-AdG20100224). The data reported in this paper can be made available by contacting the corresponding authors. We thank J. Middelburg and H. Røy for thoughtful comments on this work.
Author information
Authors and Affiliations
Contributions
All FTR experiments were performed by M.F.B. and supervised by P.L.M.C., who were both jointly responsible for the experimental design and research direction, with significant input from R.N.G. and J.B. Effluent volatile fatty acid analysis was performed by M.F.B. and supervised by P.J.M. Metabolomics analysis was performed by H.H.-S. Algal culture experiments were performed by M.K. and M.F.B. and supervised by J.B. and P.L.M.C. The manuscript was written by M.F.B. and P.L.M.C. All authors contributed to discussion and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 480 kb)
Rights and permissions
About this article
Cite this article
Bourke, M., Marriott, P., Glud, R. et al. Metabolism in anoxic permeable sediments is dominated by eukaryotic dark fermentation. Nature Geosci 10, 30–35 (2017). https://doi.org/10.1038/ngeo2843
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2843
This article is cited by
-
Biogeochemical properties of blue carbon sediments influence the distribution and monomer composition of bacterial polyhydroxyalkanoates (PHA)
Biogeochemistry (2023)
-
Hydrodynamic disturbance controls microbial community assembly and biogeochemical processes in coastal sediments
The ISME Journal (2022)
-
Intracellular nitrate storage by diatoms can be an important nitrogen pool in freshwater and marine ecosystems
Communications Earth & Environment (2022)
-
Metabolic flexibility allows bacterial habitat generalists to become dominant in a frequently disturbed ecosystem
The ISME Journal (2021)
-
Bacterial fermentation and respiration processes are uncoupled in anoxic permeable sediments
Nature Microbiology (2019)