Abstract
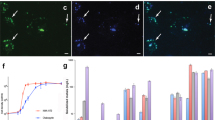
Platinum-group elements are strategically important metals1. Finding new deposits is becoming increasingly difficult owing to our limited understanding of the processes that affect their mobility in surface environments2. Microorganisms have been shown to promote the mobility of metals around ore deposits3. Here we show that microorganisms influence the mobility of platinum-group elements in mineral grains collected from Brazil, Australia and Colombia. Scanning electron microscopy showed biofilms covering the platinum-group mineral grains. The biofilms contained abundant platinum-group element nanoparticles and microcrystalline aggregates, and were dominated by Proteobacteria, many of which were closely related to known metal-resistant species. Some platinum-group mineral grains contained carbon, nitrogen, sulfur, selenium and iodine, suggesting the grains may be biogenic in origin. Molecular analyses show that Brazilian platinum–palladium grains hosted specific bacterial communities, which were different in composition from communities associated with gold grains, or communities in surrounding soils and sediments. Nano-phase metallic platinum accumulated when a metallophillic bacterium was incubated with a percolating platinum-containing medium, suggesting that biofilms can cause the precipitation of mobile platinum complexes. We conclude that biofilms are capable of forming or transforming platinum-group mineral grains, and may play an important role for platinum-group element dispersion and re-concentration in surface environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brenan, J. M. The platinum-group elements: “admirably adapted” for science and industry. Elements 4, 227–232 (2008).
Mudd, G. M. Key trends in the resource sustainability of platinum group elements. Ore Geol. Rev. 46, 106–117 (2012).
Southam, G. & Saunders, J. A. Geomicrobiology in contemporary natural systems: implications for economic geology. Econ. Geol. 100, 1067–1084 (2005).
Reith, F., Campbell, S. G., Ball, A. S., Pring, A. & Southam, G. Platinum in Earth surface environments. Earth Sci. Rev. 131, 1–21 (2014).
Reith, F., Brugger, J., Zammit, C. M., Nies, D. H. & Southam, G. Geobiological cycling of gold: from fundamental process understanding to exploration solutions. Minerals 3, 367–394 (2013).
Reith, F. et al. Nanoparticle factories: biofilms hold the key to gold dispersion and nugget formation. Geology 38, 843–846 (2010).
Shuster, J. & Southam, G. The in-vitro ‘growth’ of gold grains. Geology 43, 79–82 (2015).
Ta, C. et al. Effect of manganese oxide minerals and complexes on gold mobilization and speciation. Chem. Geol. 407, 10–20 (2015).
Wood, S. A., Mountain, B. W. & Pan, P. The aqueous geochemistry of Pt, Pd and Au: recent experimental constraints and a re-evaluation of theoretical predictions. Can. Mineral. 30, 955–982 (1992).
Fairbrother, L. et al. Biomineralization of gold in biofilms of Cupriavidus metallidurans. Environ. Sci. Technol. 47, 2628–2635 (2013).
Hussak, E. Über das Vorkommen von Pd und Platin in Brasilien. Sitzungsber. Kaiserl. Akad. Wiss., Wien, Math.-Naturwiss. 113, 379–466 (1904).
Fleet, M. H., Almeida, C. M. & Angeli, N. Botryoidal platinum, palladium and potarite from the Bom Sucesso stream, Minas Gerais, Brazil: compositional zoning and origin. Can. Mineral. 40, 341–355 (2002).
Cabral, A. R. et al. Iodine in alluvial Pt–Pd nuggets: evidence for biogenic precious-metal fixation. Chem. Geol. 281, 125–132 (2011).
Wacey, D. et al. Uncovering framboidal pyrite biogenicity using nano-scale CNorg mapping. Geology 43, 27–30 (2015).
Wacey, D. et al. Use of NanoSIMS in the search for early life on Earth: ambient inclusion trails in a c. 3400 Ma sandstone. J. Geol. Soc. 165, 43–53 (2011).
Wiesemann, N. et al. Influence of copper resistance determinants on gold transformation by Cupriavidus metallidurans strain CH34. J. Bacteriol. 195, 2298–2308 (2013).
Ward, N. L. et al. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl. Environ. Microbiol. 75, 2046–2056 (2009).
Selenska-Pobell, S. in Interactions of Microorganisms with Radionuclides (eds Keith-Roach, M. J. & Livens, F. R.) 225–254 (Elsevier Sciences, 2002).
Reith, F. et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl Acad. Sci. USA 106, 17757–17762 (2009).
Johnston, C. W. et al. 2013. Gold biomineralization by a secondary metabolite from a gold-associated microbe. Nature Chem. Biol. 9, 241–243 (2013).
Carrano, C. J. et al. Coordination chemistry of the carboxylate type siderophore rhizoferrin? The iron(III) complex and its metal analogs. Inorg. Chem. 35, 6429–6436 (1996).
Campbell, S. G. et al. Surface transformations of platinum grains from Fifield, New South Wales, Australia. Am. Mineral. 100, 1236–1243 (2015).
Oberthuer, T., Weiser, T. W., Melcher, F., Gast, L. & Woehrl, C. Detrital platinum-group minerals in rivers draining the Great Dyke, Zimbabwe. Can. Mineral. 51, 197–222 (2013).
Bottrill, R. S. in Geological Evolution of Tasmania Geological Society of Australia Special Publication Section 4.4.7, 24 (eds Corbett, K. D., Quilty, P. G. & Calver, C. R.) 141–145 (Geological Society of Australia, 2014).
Hillis, D. M. & Bull, J. J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 42, 182–192 (1993).
Clarke, K. R. & Warwick, R. M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation (Primer-E, 2001).
Anderson, M. J., Gorley, R. N. & Clarke, K. R. PERMANOVA+for PRIMER—Guide to Software and Statistical Methods (Primer-E, 2008).
Miles, A. A. & Misra, S. S. The estimation of the bactericidal power of the blood. J. Hyg. 38, 732–748 (1938).
Wirth, R. Focused ion beam (FIB) combined with SEM and TEM: advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale. Chem. Geol. 261, 217–229 (2009).
Hineman, A. & Stephan, C. Effect of dwell time on single particle inductively coupled plasma mass spectrometry data acquisition quality. J. Anal. Atom. Spectrom. 29, 1252–1257 (2014).
Acknowledgements
We acknowledge the Australian Research Council (DP1093238 to F.R.). We thank T. Reith, the Pope family (Barry, Marnie and Michael), R. Wolfe and R. Morley for their enthusiastic field support; A. R. Cabral and B. Lehmann are thanked for providing motivation and initial field support; we also thank L. Green and A. Basak (Adelaide Microscopy) for support using the FIB-SEM and J. Young (Flinders Analytical) for support with the SP-ICP-MS. Published with permission of the Director of Mines, Tasmania.
Author information
Authors and Affiliations
Contributions
F.R. conceived and led the project. C.M.Z., S.S.S., B.E., R.B., G.S. C.T., M.K., T.O., A.S.B. and J.B. contributed samples, experimental and analytical data. F.R. wrote the paper and all co-authors contributed by commenting on and revising it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 15693 kb)
Rights and permissions
About this article
Cite this article
Reith, F., Zammit, C., Shar, S. et al. Biological role in the transformation of platinum-group mineral grains. Nature Geosci 9, 294–298 (2016). https://doi.org/10.1038/ngeo2679
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2679
This article is cited by
-
Weathering of PGE sulfides and Pt–Fe alloys in the Freetown Layered Complex, Sierra Leone
Mineralium Deposita (2017)
-
Supergene neoformation of Pt-Ir-Fe-Ni alloys: multistage grains explain nugget formation in Ni-laterites
Mineralium Deposita (2017)