Abstract
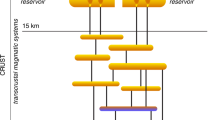
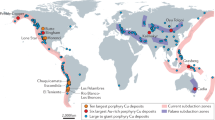
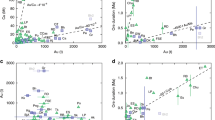
Porphyry copper deposits—the primary source of the world’s copper—are a consequence of the degassing of intrusion complexes in magmatic arcs associated with ancient subduction zones1,2. They are characterized by copper and iron sulphides, commonly found with anhydrite (CaSO4), over scales of several kilometres through intensely altered and fractured rocks1. The magmatic source of the metals is broadly understood, but the processes that transport and deposit the metals at the megaton scale are unclear. The hydrogen sulphide necessary for metal deposition is commonly assumed to form by a reaction between sulphur dioxide and water, but this reaction is inefficient3 and cannot explain the formation of economic-grade deposits. Here we use high-temperature laboratory experiments to show that a very rapid chemisorption reaction occurs between sulphur dioxide gas, a principal component of magmatic gas mixtures, and calcic feldspar, an abundant mineral in the arc crust. The chemisorption reaction generates the mineral anhydrite and hydrogen sulphide gas, and triggers deposition of metal sulphides. We use thermodynamic calculations to show that as magmatic gas cools and expands the concentration of hydrogen sulphide gas increases exponentially to drive efficient deposition of metal sulphides and consequent formation of economic-grade porphyry copper deposits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sillitoe, R. H. Porphyry copper systems. Econ. Geol. 105, 3–41 (2010).
Richards, J. P. Postsubduction porphyry Cu–Au and epithermal Au deposits: Products of remelting of subduction-modified lithosphere. Geology 37, 247–250 (2009).
Giggenbach, W. F. Redox processes governing the chemistry of fumarolic gas discharges from White Island, New Zealand. Appl. Geochem. 2, 143–161 (1987).
Singer, D. A., Berger, V. I. & Moring, B. C. Porphyry Copper Deposits of the World: Database, Maps, and Preliminary Analysis (US Department of the Interior, US Geological Survey, 2008).
Landtwing, M. R. et al. The Bingham Canyon porphyry Cu–Mo–Au deposit. III. Zoned copper–gold ore deposition by magmatic vapor expansion. Econ. Geol. 105, 91–118 (2010).
Henley, R. W. & McNabb, A. Magmatic vapor plumes and ground-water interaction in porphyry copper emplacement. Econ. Geol. 73, 1–20 (1978).
Weis, P., Dreisner, T. & Heinrich, C. A. Porphyry-copper ore shells form at stable pressure–temperature fronts within dynamic fluid plumes. Science 338, 1613–1616 (2012).
Oppenheimer, C., Fischer, T. P. & Scaillet, B. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 111–179 (Elsevier, 2014).
Nadeau, O., Williams-Jones, A. E. & Stix, J. Sulphide magma as a source of metals in arc-related magmatic hydrothermal ore fluids. Nature Geosci. 3, 501–505 (2010).
Henley, R. W. & Berger, B. R. Nature’s refineries—Metals and metalloids in arc volcanoes. Earth-Sci. Rev. 125, 146–170 (2013).
Wilkinson, J. J. Triggers for the formation of porphyry ore deposits in magmatic arcs. Nature Geosci. 6, 917–925 (2013).
Gustafson, L. B. & Hunt, J. P. The porphyry copper deposit at El Salvador, Chile. Econ. Geol. 70, 857–912 (1975).
Cooke, D. R., Hollings, P., Wilkinson, J. J. & Tosdal, R. M. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 357–381 (Elsevier, 2014).
Sillitoe, R. H. Economic Geology One Hundredth Anniversary Volume 724–768 (Society of Economic Geologists, 2005).
Dickson, F. W., Blount, C. W. & Tunell, G. Use of hydrothermal solution equipment to determine the solubility of anhydrite in water from 100 °C to 275 °C and from 1 bar to 1000 bars pressure. Am. J. Sci. 261, 61–78 (1963).
Burnham, C. W. in Geochemistry of Hydrothermal Ore Deposits (ed. Barnes, H. L.) 71–136 (John Wiley, 1979).
Li, E. Y., Chareev, D. A., Shilobreeva, S. N., Grichuk, D. V. & Tyutyunnik, O. A. Experimental study of sulfur dioxide interaction with silicates and aluminosilicates at temperatures of 650 and 850 °C. Geochem. Int. 48, 1039–1046 (2010).
Fegley, B. & Prinn, R. G. Estimation of the rate of volcanism on Venus from reaction rate measurements. Nature 337, 55–58 (1989).
Ayris, P. M. et al. SO2 sequestration in large volcanic eruptions: High-temperature scavenging by tephra. Geochim. Cosmochim. Acta 110, 58–69 (2013).
Lee, R. J., King, P. L. & Ramsey, M. S. Spectral analysis of synthetic quartzofeldspathic glasses using laboratory thermal infrared spectroscopy. J. Geophys. Res. 115, B06202 (2010).
Ohmoto, H. & Goldhaber, M. B. Geochemistry of Hydrothermal Ore Deposits 517–612 (John Wiley, 1997).
Kesler, S. E., Chryssoulis, S. L. & Simon, G. Gold in porphyry copper deposits: Its abundance and fate. Ore Geol. Rev. 21, 103–124 (2002).
Bodnar, R. J., Lecumberri-Sanchez, P., Moncada, D. & Steele-MacInnis, M. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 119–142 (Elsevier, 2014).
Chambefort, I., Dilles, J. H. & Kent, A. J. R. Anhydrite-bearing andesite and dacite as a source for sulfur in magmatic-hydrothermal mineral deposits. Geology 36, 719–722 (2008).
Mastin, L. G. & Ghiorso, M. S. Adiabatic temperature changes of magma–gas mixtures during ascent and eruption. Contrib. Mineral. Petrol. 141, 307–321 (2001).
Migdisov, A. A., Bychkov, A. Y., Williams-Jones, A. E. & van Hinsberg, V. J. A predictive model for the transport of copper by HCl-bearing water vapour in ore-forming magmatic-hydrothermal systems: Implications for copper porphyry ore formation. Geochim. Cosmochim. Acta 129, 33–53 (2014).
Audétat, A. & Pettke, T. Evolution of a porphyry-Cu mineralized magma system at Santa Rita, New Mexico (USA). J. Petrol. 47, 2021–2046 (2006).
Herve, M. et al. in Geology and Genesis of Major Copper Deposits and Districts of the World (eds Hedenquist, J. W., Harris, M. & Camus, F.) 55–78 (Society of Economic Geologists, 2012).
Meinert, L. D., Hefton, K. K., Mayes, D. & Tasiran, I. Geology, zonation, and fluid evolution of the Big Gossan Cu–Au skarn deposit, Ertsberg District, Irian Jaya. Econ. Geol. 92, 509–534 (1997).
Carroll, M. R. & Wyllie, P. J. Experimental phase relations in the system tonalite-peridotite-H2O at 15 kb; implications for assimilation and differentiation processes near the crust–mantle boundary. J. Petrol. 30, 1351–1382 (1989).
Acknowledgements
This investigation was triggered by interactions with P. Delmelle and P. Ayris in relation to the reactivity of volcanic ashes. We thank R. Arculus, M. Goldhaber, R. King, R. Sillitoe, H. O’Neill, A. Putnis, J. Ward and T. Whan for valuable discussions. We also wish to acknowledge the foundations of porphyry copper analysis laid by L. Gustafson. We thank N. Bishop, D. Olson and K. Schroeder of Kennecott Exploration (Rio Tinto) for confirmation of the occurrence of anhydrite in deep drilling at Bingham Canyon, Utah. K. Friehauf very kindly provided the photograph of the Grasberg porphyry copper deposit for Fig. 1a. Graphic enhancement was provided by D. Henley, and D. Hill kindly provided his original cartoon for a generic volcano that we have used in Fig. 1c. Constructive comments on earlier versions of this work were received from J. Dilles and R. Herrington. Funding was provided by an Australian Research Council Future Fellowship to P.L.K. R.W.H. and P.L.K. wish to dedicate this paper to the late W. S. Fyfe, one of the founders of modern geochemistry, and a lifetime friend and mentor.
Author information
Authors and Affiliations
Contributions
R.W.H. conceived the initial concept. All authors collaborated in the analysis and interpretation and the growth of the concept.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 17186 kb)
Rights and permissions
About this article
Cite this article
Henley, R., King, P., Wykes, J. et al. Porphyry copper deposit formation by sub-volcanic sulphur dioxide flux and chemisorption. Nature Geosci 8, 210–215 (2015). https://doi.org/10.1038/ngeo2367
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2367
This article is cited by
-
Copper sulfide deposition and remobilisation triggered by non-magmatic fluid incursion in the single-intrusion Tongchang porphyry system, SE China
Scientific Reports (2024)
-
Combined Feldspar-Destructive Processes and Hypogene Sulfide Mineralization in the Porphyry Copper Systems: Potentials for Geochemical Signals of Ore Discovering
Iranian Journal of Science and Technology, Transactions A: Science (2022)
-
Crustal magmatic controls on the formation of porphyry copper deposits
Nature Reviews Earth & Environment (2021)
-
Reduction of oxidized sulfur in the formation of the Grasberg porphyry copper-gold deposit, Papua, Indonesia
Mineralium Deposita (2021)
-
An experimental study of SO2 reactions with silicate glasses and supercooled melts in the system anorthite–diopside–albite at high temperature
Contributions to Mineralogy and Petrology (2019)