Abstract
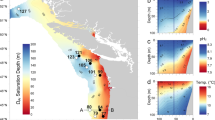
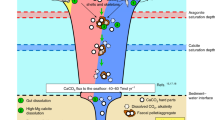
The carbonate chemistry of the surface ocean is rapidly changing with ocean acidification, a result of human activities1. In the upper layers of the Southern Ocean, aragonite—a metastable form of calcium carbonate with rapid dissolution kinetics—may become undersaturated by 2050 (ref. 2). Aragonite undersaturation is likely to affect aragonite-shelled organisms, which can dominate surface water communities in polar regions3. Here we present analyses of specimens of the pteropod Limacina helicina antarctica that were extracted live from the Southern Ocean early in 2008. We sampled from the top 200 m of the water column, where aragonite saturation levels were around 1, as upwelled deep water is mixed with surface water containing anthropogenic CO2. Comparing the shell structure with samples from aragonite-supersaturated regions elsewhere under a scanning electron microscope, we found severe levels of shell dissolution in the undersaturated region alone. According to laboratory incubations of intact samples with a range of aragonite saturation levels, eight days of incubation in aragonite saturation levels of 0.94–1.12 produces equivalent levels of dissolution. As deep-water upwelling and CO2 absorption by surface waters is likely to increase as a result of human activities2,4, we conclude that upper ocean regions where aragonite-shelled organisms are affected by dissolution are likely to expand.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D. & Hales, B. Evidence for upwelling of corrosive ‘acidified’ water onto the continental shelf. Science 320, 1490–1492 (2008).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Hunt, B. P. V. et al. Pteropods in Southern Ocean ecosystems. Prog. Oceanogr. 78, 193–221 (2008).
Le Quere, C. et al. Saturation of the Southern Ocean CO2 sink due to recent climate change. Science 316, 1735–1738 (2007).
Fabry, V. J. Shell growth rates of pteropod and heteropod molluscs and aragonite production in the open ocean: Implications for the marine carbonate system. J. Mar. Res. 48, 209–222 (1990).
Broecker, W. S. & Takahashi, T. in The Fate of Fossil Fuel CO2 in the Oceans (eds Andersen, N. R. & Malahoff, A.) 213–241 (1977).
Betzer, P. R. et al. The oceanic carbonate system—a reassessment of biogenic control. Science 226, 1074–1077 (1984).
Feely, R. A. et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (2004).
Byrne, R. H., Acker, J. G., Betzer, P. R., Feely, R. A. & Cates, M. H. Water column dissolution of aragonite in the Pacific Ocean. Nature 312, 321–326 (1984).
Feely, R. A. et al. Winter-summer variations of calcite and aragonite saturation in the Northeast Pacific. Mar. Chem. 25, 227–241 (1988).
Yamamoto-Kawai, M., McLaughlin, F. A., Carmack, E. C., Nishino, S. & Shimada, K. Aragonite undersaturation in the Arctic Ocean: Effects of ocean acidification and sea ice melt. Science 326, 1098–1100 (2009).
McNeil, B. I. & Matear, R. J. Southern Ocean acidification: A tipping point at 450 ppm atmospheric CO2 . Proc. Natl Acad. Sci. USA 105, 18860–18864 (2008).
Steinacher, M., Joos, F., Frolicher, T. L., Plattner, G. K. & Doney, S. C. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences 6, 515–533 (2009).
Comeau, S., Jeffree, R., Teyssie, J. L. & Gattuso, J. P. Response of the Arctic pteropod Limacina helicina to projected future environmental conditions. PLoS ONE 5, e11362 (2010).
Comeau, S., Gorsky, G., Alliouane, S. & Gattuso, J. P. Larvae of the pteropod Cavolinia inflexa exposed to aragonite undersaturation are viable but shell-less. Mar. Biol. 157, 2341–2345 (2010).
Roberts, D. et al. Interannual pteropod variability in sediment traps deployed above and below the aragonite saturation horizon in the Sub-Antarctic Southern Ocean. Polar Biol. 34, 1739–1750 (2011).
Heywood, K. J., Garabato, A. C. N. & Stevens, D. P. High mixing rates in the abyssal Southern Ocean. Nature 415, 1011–1014 (2002).
Kahru, M., Mitchell, B. G., Gille, S. T., Hewes, C. D. & Holm-Hansen, O. Eddies enhance biological production in the Weddell-Scotia confluence of the Southern Ocean. Geophys. Res. Lett. 34, L14603 (2007).
Park, J., Ohb, I-S., Kim, H-C. & Yoo, S. Variability of SeaWiFs chlorophyll-a in the southwest Atlantic sector of the Southern Ocean: Strong topographic effects and weak seasonality. Deep-Sea Res Pt. I 57, 604–620 (2010).
Jones, E., Bakker, D., Venables, H. & Watson, A. Dynamic seasonal cycling of inorganic carbon downstream of South Georgia, Southern Ocean. Deep-Sea Res. Pt. II 59–60, 25–35 (2012).
Bednarsek, N., Tarling, G., Fielding, S. & Bakker, D. Population dynamics and biogeochemical significance of Limacina helicina antarctica in the Scotia Sea (Southern Ocean). Deep-Sea Res. Pt. II 59–60, 105–116 (2012).
Jansen, H., Zeebe, R.E. & Wolf-Gladrow, D.A. Modeling the dissolution of settling CaCO3 in the ocean. Glob. Biogeochem. Cycles 16, 1027 (2002).
Francois, R., Honjo, S., Krishfield, R. & Manganini, S. Factors controlling the flux of organic carbon to the bathypelagic zone of the ocean. Glob. Biogeochem. Cycles 16, 1087 (2002).
Fabry, V. J., McClintock, J. B., Mathis, J. T. & Grebmeier, J. M. Ocean acidification at high latitudes: The bell weather. Oceanography 22, 160–171 (2009).
Johnson, K. M., Sieburth, J. M., Williams, P. J. L. & Brandstrom, L. Coulometric total carbon dioxide analysis for marine studies—automation and calibration. Mar. Chem. 21, 117–133 (1987).
Dickson, A. G. An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data. Deep-Sea Res. 28, 609–623 (1981).
Lewis, E. & Wallace, D. W. R. co2sys - Program Developed for CO 2 System Calculations Report ORNL/CDIAC-105 (Carbon Dioxide Information and Analysis Centre, Oak Ridge Natl. Lab., US Dep. of Energy, 1998).
Mehrbach, C., Culberson, C. H., Hawley, J. E. & Pytkowicz, R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973).
Dickson, A. G. & Millero, F. J. A comparison of the equilibrium constants for the dissociationof carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743 (1987).
Bednarsek, N. et al. Description and quantification of pteropod shell dissolution: A sensitive bioindicator of ocean acidification. Global Change Biol. 18, 2378–2388 (2012).
Acknowledgements
This work was supported by the FAASIS (Fellowships in Antarctic Air-Sea-Ice Science), a Marie Curie Early Stage Training Network awarded to N.B. G.A.T., S.F. and P.W. carried out this work as part of the Ecosystems core-science programme at the British Antarctic Survey. G.A.T., P.W. and D.B. received further support during the analysis and synthesis stages from the pelagic consortium of the UK Ocean Acidification programme, funded by NERC, Defra and DECC (grant no. NE/H017267/1). D. McCorkle and A. Cohen of Woods Hole Oceanographic Institution helped develop a shell preparation method and commented on previous drafts of the manuscript. Image analysis was carried out at the University of East Anglia with the assistance of R. Montagna. Sampling operations were supported by the officers and crew of the RRS James Clark Ross with net-sampling equipment support from P. Enderlein of the British Antarctic Survey. P. Bucktrout assisted with graphical presentations.
Author information
Authors and Affiliations
Contributions
G.A.T. and D.C.E.B. conceived the project; N.B. carried out the fieldwork, with the assistance of G.A.T., S.F. and P.W.; E.M.J. and H.J.V. provided supporting environmental data; A.K. helped develop a method of shell preparation for SEM analysis; B.L. developed an image analysis method; G.A.T., N.B. and D.C.E.B. co-wrote the manuscript, with theoretical overviews provided by R.A.F. and all remaining authors commenting.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1918 kb)
Supplementary Movie
Supplementary Movie (MOV 19156 kb)
Rights and permissions
About this article
Cite this article
Bednaršek, N., Tarling, G., Bakker, D. et al. Extensive dissolution of live pteropods in the Southern Ocean. Nature Geosci 5, 881–885 (2012). https://doi.org/10.1038/ngeo1635
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1635
This article is cited by
-
Severe 21st-century ocean acidification in Antarctic Marine Protected Areas
Nature Communications (2024)
-
Malformation in coccolithophores in low pH waters: evidences from the eastern Arabian Sea
Environmental Science and Pollution Research (2023)
-
The variability in abundance and shell size of the thecosome pteropods Limacina spp. in the seasonal ice zone of the Southern Ocean in March
Polar Biology (2023)
-
Pteropods make thinner shells in the upwelling region of the California Current Ecosystem
Scientific Reports (2021)
-
Tracking coastal acidification from erosion of gastropod shells: spatial sensitivity and organism size effect
Environmental Monitoring and Assessment (2021)