Abstract
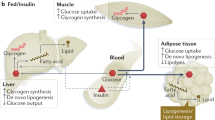
Impaired insulin action is a key feature of type 2 diabetes and is also found, to a more extreme degree, in familial syndromes of insulin resistance. Although inherited susceptibility to insulin resistance may involve the interplay of several genetic loci, no clear examples of interactions among genes have yet been reported. Here we describe a family in which five individuals with severe insulin resistance, but no unaffected family members, were doubly heterozygous with respect to frameshift/premature stop mutations in two unlinked genes, PPARG and PPP1R3A these encode peroxisome proliferator activated receptor γ, which is highly expressed in adipocytes, and protein phosphatase 1, regulatory subunit 3, the muscle-specific regulatory subunit of protein phosphatase 1, which are centrally involved in the regulation of carbohydrate and lipid metabolism, respectively. That mutant molecules primarily involved in either carbohydrate or lipid metabolism can combine to produce a phenotype of extreme insulin resistance provides a model of interactions among genes that may underlie common human metabolic disorders such as type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
12 August 2002
The word 'compound' was changed to 'doubly'; the full text was updated, the pdf was appended with a note. An erratum will be published in the September issue.
Notes
NOTE: Owing to a copy-editing error that was implemented after the authors returned the corrected proofs, the term 'doubly heterozygous' was substituted with the term 'compound heterozygous' throughout the text and in Table 1. Similarly, 'double heterozygotes' was erroneously substituted with 'compound heterozygotes'. The full text of the online version of the Letter, including Table 1, has been corrected. Per company policy, the PDF version has not been corrected; an erratum will be published in an upcoming issue. Nature Genetics sincerely regrets these errors.
References
Barroso, I. et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402, 880–883 (1999).
Rosen, E.D. & Spiegelman, B.M. Pparγ: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 276, 37731–37734 (2001).
Mukherjee, R. et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 386, 407–410 (1997).
Olefsky, J.M. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J. Clin. Invest. 106, 467–472 (2000).
Yamauchi, T. et al. Inhibition of RXR and PPARγ ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 108, 1001–1013 (2001).
Kubota, N. et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. 4, 597–609 (1999).
Miles, P.D., Barak, Y., He, W., Evans, R.M. & Olefsky, J.M. Improved insulin-sensitivity in mice heterozygous for PPAR-γ deficiency. J. Clin. Invest. 105, 287–292 (2000).
Bjorntorp, P. and Sjostrom, L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism 27, 1853–1865 (1978).
Newgard, C.B., Brady, M.J., O'Doherty, R.M. & Saltiel, A.R. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49, 1967–1977 (2000).
Suzuki, Y., et al. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific phosphatase PP1G/RGL. Mol. Cell. Biol. 21, 2683–2694 (2001).
Nielsen, J.N. et al. Glycogen synthase localization and activity in rat skeletal muscle is strongly dependent on glycogen content. J. Physiol. 531, 757–769 (2001).
Burghes, A.H., Vaessin, H.E. & de La Chapelle, A. Genetics. The land between Mendelian and multifactorial inheritance. Science 293, 2213–2214 (2001).
Bruning, J.C. et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88, 561–572 (1997).
Birnbaum, M.J. Diabetes. Dialogue between muscle and fat. Nature 409, 672–673 (2001).
Abel, E.D. et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 (2001).
Steppan, C.M. & Lazar, M.A. Resistin and obesity-associated insulin resistance. Trends Endocrinol. Metab. 13, 18–23 (2002).
Kelley, D.E. & Goodpaster, B.H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24, 933–941 (2001).
McGarry, J.D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258, 766–770 (1992).
Zhang, L. et al. Whole genome amplification from a single cell: implications for genetic analysis. Proc. Natl Acad. Sci. USA 89, 5847–5851 (1992).
Thorpe, K.L., Schafer, A.J., Genin, E., Trowsdale, J. & Beck, S. Detection of polymorphism in the RING3 gene by high-throughput fluorescent SSCP analysis. Immunogenetics 49, 256–265 (1999).
Collingwood, T.N., Adams, M., Tone, Y. & Chatterjee, V.K. Spectrum of transcriptional, dimerization, and dominant negative properties of twenty different mutant thyroid hormone β-receptors in thyroid hormone resistance syndrome. Mol. Endocrinol. 8, 1262–1277 (1994).
Zamir, I., Zhang, J. & Lazar, M.A. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 11, 835–846 (1997).
Forman, B.M. et al. 15-Deoxy-Δ12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83, 803–812 (1995).
Rasmussen, S.K. et al. Adenovirus-mediated expression of a naturally occurring Asp905Tyr variant of the glycogen-associated regulatory subunit of protein phosphatase-1 in L6 myotubes. Diabetologia 43, 718–722 (2000).
Rico-Sanz, J. et al. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J. Appl. Physiol. 87, 2068–2072 (1999).
Tang, P.M., Bondor, J.A., Swiderek, K.M. & DePaoli-Roach, A.A. Molecular cloning and expression of the regulatory (RGI) subunit of the glycogen-associated protein phosphatase. J. Biol. Chem. 266, 15782–15789 (1991).
Thomas, E.L. et al. Magnetic resonance imaging of total body fat. J. Appl. Physiol. 85, 1778–1785 (1998).
Black, D. et al. Obesity. A report of the Royal College of Physicians. J. R. Coll. Physicians Lond. 17, 5–65 (1983).
Matthews, D.R. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Acknowledgements
This study drew upon the combined efforts of many individuals at Incyte Genomics Cambridge, to whom we extend our appreciation. We thank the patients and their families who participated in this study, P. Luzio for helpful discussion, V. Ibbotson for assistance with clinical investigations, K. Ong for HOMA analysis and I. Halsall for insulin and leptin measurements. D.B.S. is a Wellcome Trust Training Fellow, and S.O'R. and V.K.K.C. are supported by the Wellcome Trust. A.M. is funded by an Individual Marie Curie Fellowship. N.J.W., A.H.H., E.L.T. and J.D.B. are supported by the Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Savage, D., Agostini, M., Barroso, I. et al. Digenic inheritance of severe insulin resistance in a human pedigree. Nat Genet 31, 379–384 (2002). https://doi.org/10.1038/ng926
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng926
This article is cited by
-
PPARγ and Diabetes: Beyond the Genome and Towards Personalized Medicine
Current Diabetes Reports (2021)
-
Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease
Human Genetics (2013)
-
IRS2 variants and syndromes of severe insulin resistance
Diabetologia (2009)
-
Molekulare Diagnostik des Diabetes mellitus
Der Internist (2006)
-
Sequencing EVC and EVC2 identifies mutations in two-thirds of Ellis–van Creveld syndrome patients
Human Genetics (2006)