Abstract
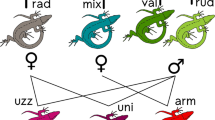
Green toads are common in the Palaearctic region, where they have differentiated into several taxa1,2. The toads exist with variable amounts of ploidy, similar to other anuran species3 or reptiles4. In vertebrate biology, the very rare occurrence of triploidy is coupled with infertility or unisexuality, or requires the coexistence of individuals of different ploidy in a reproductive community. The reproduction of naturally occurring triploids has been reported to occur only through parthenogenesis, gynogenesis or hybridogenesis. The bisexual reproduction of pure triploids has been considered to be impossible because of the problem of equally distributing three chromosome sets in meiosis. Here we report geographically isolated populations of green toads (Bufo viridis complex) that are all-triploid and reproduce bisexually.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Borkin, L.J. in Patterns of Distribution of Amphibians (ed. Duellman, W.E.) 329–420 (J. Hopkins Univ. Press, Baltimore, 1999).
Stöck, M., Günther, R. & Böhme, W. Progress towards a taxonomic revision of the Asian Bufo viridis group: current status of nominal taxa and unsolved problems. Zool. Abh. Mus. Tierkd. Dresden 51, 253–319 (2001).
Green, D.S. & Sessions, K. (eds) Amphibian Cytogenetics and Evolution (Harcourt Brace Jovanovich, San Diego, 1991).
Bickham, J.W., Hanks, B.G., Hale, D.W. & Martin, J.E. Ploidy diversity and the production of balanced gametes in male twist-necked turtles (Platemys platycephala). Copeia 1993, 723–727 (1993).
Roth, P. & Ráb, P. in Proc. 4th Ord. Gen. Meeting Soc. Europ. Herpetol. (eds van Gelder, J.J., Strijbosch, H. & Bergers, P.J.M.) 335–338 (Nijmegen, 1987).
Stöck, M. Polyploidy and Speciation in the Bufo viridis Complex. Thesis, Martin-Luther-Univ. Halle-Wittenberg (2001).
Castellano, S., Giacoma, C., Dujsebayeva, T., Odierna, G. & Balletto, E. Morphometrical and acoustical comparison between diploid and tetraploid green toads. Biol. J. Linn. Soc. 63, 257–281 (1998).
Mezhzherin, S.V. & Pisanets, E.M. Genetic structure and origin of the tetraploid toad Bufo danatensis Pisanets 1978 (Amphibia, Bufonidae) from Central Asia: description of biochemical polymorphism and comparison of heterozygosity levels in diploid and tetraploid species. Genetica 31, 43–53 (1995).
Pisanets, E.M. On the new species Bufo danatensis sp. n. from Turkmenistan. Doklady Akademii Nauk Ukrainskoi SSR B 3, 280–278 (1978).
Borkin, L.Y. et al. On the distribution of diploid, triploid and tetraploid green toads in south-eastern Kazakhstan. Russ. J. Herpetol. 8, 45–53 (2001).
Stöck, M., Schmid, M., Steinlein, C. & Grosse, W.-R. Mosaicism in somatic triploid specimens of the Bufo viridis complex in the Karakoram with examination of calls, morphology and taxonomic conclusions. Ital. J. Zool. 66, 215–232 (1999).
Schartl, M. et al. On the stability of dispensable constituents of the eukaryotic genome: stability of coding sequences versus truly hypervariable sequences in a clonal vertebrate, the amazon molly Poecilia formosa. Proc. Natl Acad. Sci. USA 88, 8759–8763 (1991).
Chen, H. & Leibenguth, F. Studies on multilocus fingerprints, RAPD markers and mitochondrial DNA of a gynogenetic fish (Carassius auratus gibelio). Biochem. Gen. 33, 297–306 (1995).
Stöck, M. & Grosse, W.-R. Erythrocyte size and ploidy determination in green toads (Bufo viridis complex) from Middle Asia. Alytes (Paris) 15, 72–90 (1997).
Günther, R., Uzzell, T. & Berger, L. Inheritance patterns in triploid Rana “esculenta” (Amphibia, Salientia). Mitt. Zool. Mus. Berl. 55, 35–57 (1979).
Vinogradov, A.E., Borkin, L.J., Günther, R. & Rosanov, J.M. Genome elimination in diploid and triploid Rana esculenta males: cytological evidence from DNA flow cytometry. Genome 33, 619–627 (1990).
Alves, M.J., Coelho, M.M. & Collares-Pereira, M.J. Diversity in the reproductive modes of females of the Rutilus alburnoides complex (Teleostei, Cyprinidae): a way to avoid the genetic constraints of uniparentalism. Mol. Biol. Evol. 15, 1233–1242 (1998).
Carmona, J.A., Sanjur, O.I., Doadrio, I., Machordom, A. & Vrijenhoek, R.C. Hybridogenetic reproduction and maternal ancestry of polyploid Iberian fish: the Tropidophoxinellus alburnoides complex. Genetics 146, 983–993 (1997).
Ohtani, H. Mechanism of chromosome elimination in the hybridogenetic spermatogenesis of allotriploid males between Japanese and European water frogs. Chromosoma 102, 158–162 (1993).
Sumida, M. & Nishioka, M. Reproductive capacity of allotriploids between Rana tsushimensis from Tsushima and Rana japonica from Ichinoseki and Hiroshima. Sci. Rep. Lab. Amph. Biol. Hiroshima Univ. 12, 133–175 (1993).
Uzzell, T. Meiotic mechanisms of naturally occurring unisexual vertebrates. Am. Nat. 104, 433–445 (1970).
Beukeboom, L.W. & Vrijenhoek, R.C. Evolutionary genetics and ecology of sperm dependent parthenogenesis. J. Evol. Biol. 11, 755–782 (1998).
Schmid, M. Chromosome banding in Amphibia. I. Constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66, 361–388 (1978).
Klapperstück, T. & Wohlrab, W. DNA image cytometry on sections as compared with image cytometry on smears and flow cytometry in melanoma. Cytometry 25, 82–89 (1996).
Morgan, G.T., Macgregor, H.C. & Colman, A. Multiple ribosomal gene sites revealed by in situ hybridization of Xenopus rDNA to Triturus lampbrush chromosomes. Chromosoma 80, 309–330 (1980).
Gall, J.G. & Wu, Z. Lampbrush chromosomes. Methods Cell Biol. 36, 149–166 (1991).
Callan, H.G., Gall, J.G. & Berg, C.A. The lamp brush chromosomes of Xenopus laevis: preparation, identification, and distribution of 5S DNA sequences. Chromosoma 95, 236–250 (1987).
Tikel, D. et al. Polymerase chain reaction primers for polymorphic microsatellite loci in the invasive toad species Bufo marinus. Mol. Ecol. 9, 1927–1929 (2000).
Rowe, G., Beebee, T.J.C. & Burke, T. PCR primers for polymorphic microsatellite loci in the anuran amphibian Bufo calamita. Mol. Ecol. 6, 401–402 (1997).
Scribner, K.T., Arntzen, J.W. & Burke, T. Comparative analysis of intra- and interpopulation genetic diversity in Bufo bufo, using allozyme, single locus microsatellite, minisatellites and multilocus minisatellites data. Mol. Biol. Evol. 11, 737–748 (1994).
Acknowledgements
We thank W. Böhme, L.J. Borkin, A. Dubois and R. Günther for references; R. Dressel and H. Veith for assistance during field work in Pakistan; A. Hamel for combinatory views; I. Haufe for statistical advice; E. Trautmann and M. Kuhne for help with histological preparations; D. Frynta for animals; G. Moritz for technical support and C. and H. Stöck for technical and financial help. The costs of publication have been defrayed by the 15th International Chromosome Conference. This work was supported in part by the Deutsche Forschungsgemeinschaft and a graduate fellowship of the country Saxony-Anhalt (Germany; to M.St.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Stöck, M., Lamatsch, D., Steinlein, C. et al. A bisexually reproducing all-triploid vertebrate. Nat Genet 30, 325–328 (2002). https://doi.org/10.1038/ng839
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng839
This article is cited by
-
Achiasmatic meiosis in the unisexual Amazon molly, Poecilia formosa
Chromosome Research (2022)
-
Population genomics of an exceptional hybridogenetic system of Pelophylax water frogs
BMC Evolutionary Biology (2019)
-
Polyploid lineages in the genus Porphyra
Scientific Reports (2018)
-
Chromosomes in a genome-wise order: evidence for metaphase architecture
Molecular Cytogenetics (2016)
-
Post-zygotic selection against parental genotypes during larval development maintains all-hybrid populations of the frog Pelophylax esculentus
BMC Evolutionary Biology (2015)