Abstract
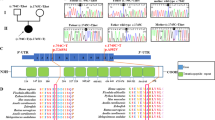
Complex III (CIII; ubiquinol cytochrome c reductase of the mitochondrial respiratory chain) catalyzes electron transfer from succinate and nicotinamide adenine dinucleotide-linked dehydrogenases to cytochrome c. CIII is made up of 11 subunits, of which all but one (cytochrome b) are encoded by nuclear DNA. CIII deficiencies are rare and manifest heterogeneous clinical presentations1,2. Although pathogenic mutations in the gene encoding mitochondrial cytochrome b have been described3,4,5,6,7, mutations in the nuclear-DNA-encoded subunits have not been reported. Involvement of various genes has been indicated in assembly of yeast CIII (refs. 8–11). So far only one such gene, BCS1L, has been identified in human12. BCS1L represents, therefore, an obvious candidate gene in CIII deficiency. Here, we report BCS1L mutations in six patients, from four unrelated families and presenting neonatal proximal tubulopathy, hepatic involvement and encephalopathy. Complementation study in yeast confirmed the deleterious effect of these mutations. Mutation of BCS1L would seem to be a frequent cause of CIII deficiency, as one-third of our patients have BCS1L mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mourmans, J. et al. Clinical heterogeneity in respiratory chain complex III deficiency in childhood. J. Neurol. Sci. 149, 111–117 (1997).
von Kleist-Retzow, J.C. et al. A high rate (20%-30%) of parental consanguinity in cytochrome-oxidase deficiency. Am. J. Hum. Genet. 63, 428–435 (1998).
Dumoulin, R. et al. A novel gly290asp mitochondrial cytochrome b mutation linked to a complex III deficiency in progressive exercise intolerance. Mol. Cell. Probes 10, 389–391 (1996).
Andreu, A.L. et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 341, 1037–1044 (1999).
Valnot, I. et al. A mitochondrial cytochrome b mutation but no mutations of nuclearly encoded subunits in ubiquinol cytochrome c reductase (complex III) deficiency. Hum. Genet. 104, 460–466 (1999).
De Coo, I.F. et al. A 4-base pair deletion in the mitochondrial cytochrome b gene associated with parkinsonism/MELAS overlap syndrome. Ann. Neurol. 45, 130–133 (1999).
Keightley, J.A. et al. Mitochondrial encephalomyopathy and complex III deficiency associated with a stop-codon mutation in the cytochrome b gene. Am. J. Hum. Genet. 67, 1400–1410 (2000).
Wu, M. & Tzagoloff, A. Identification and characterization of a new gene (CBP3) required for the expression of yeast coenzyme QH2-cytochrome c reductase. J. Biol. Chem. 264, 11122–11130 (1989).
Crivellone, M.D. Characterization of CBP4, a new gene essential for the expression of ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J. Biol. Chem. 269, 21284–21292 (1994).
Nobrega, F.G., Nobrega, M.P. & Tzagoloff, A. BCS1, a novel gene required for the expression of functional Rieske iron-sulfur protein in Saccharomyces cerevisiae. EMBO J. 11, 3821–3829 (1992).
Brasseur, G., Tron, G., Dujardin, G., Slonimski, P.P. & Brivet-Chevillotte, P. The nuclear ABC1 gene is essential for the correct conformation and functioning of the cytochrome bc1 complex and the neighbouring complexes II and IV in the mitochondrial respiratory chain. Eur. J. Biochem. 246, 103–111 (1997).
Petruzzella, V. et al. Identification and characterization of human cDNAs specific to BCS1, PET112, SCO1, COX15, and COX11, five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics 54, 494–504 (1998).
Hill, J.E., Myers, A.M., Koerner, T.J. & Tzagoloff, A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2, 163–167 (1986).
Cruciat, C.M., Hell, K., Folsch, H., Neupert, W. & Stuart, R. A. Bcs1p, an AAA-family member, is a chaperone for the assembly of the cytochrome bc(1) complex. EMBO J. 18, 5226–5233 (1999).
Zhu, Z. et al. E. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nature Genet. 20, 337–343 (1998).
Tiranti, V. et al. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 63, 1609–1621 (1998).
von Kleist-Retzow, J.C. et al. Mutations in SURF1 are not specifically associated with Leigh Syndrome. J. Med. Genet. 38,109–113 (2001).
Rahman, S., Brown, R.M., Chong, W.K., Wilson, C.J. & Brown, G.K. A SURF1 gene mutation presenting as isolated leukodystrophy. Ann. Neurol. 49, 797–800 (2001).
Papadopoulou, L.C. et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nature Genet. 23, 333–337 (1999).
Rustin, P. et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 228, 35–51 (1994).
Faye, G., Kujawa, C. & Fukuhara, H. Physical and genetic organization of petite and grande yeast mitochondrial DNA. IV. In vivo transcription products of mitochondrial DNA and localization of 23 S ribosomal RNA in petite mutants of Saccharomyces cerevisiae. J. Mol. Biol. 88, 185–203 (1974).
Mashkevich, G., Repetto, B., Glerum, D.M. & Tzagoloff, A. SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 272, 14356–14364 (1997).
Kuklin, A., Munson, K., Gjerde, D., Haefele, R. & Taylor, P. Detection of single-nucleotide polymorphisms with the WAVE DNA fragment analysis system. Genet. Test. 1, 201–206 (1997).
Gietz, R.D. & Schiestl, R.H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 7, 253–263 (1991).
von Kleist-Retzow, J.C. et al. Biochemical, genetic and immunoblot analyses of 17 patients with an isolated cytochrome c oxidase deficiency. Biochim. Biophys. Acta 1455, 35–44 (1999).
Acknowledgements
We thank J.M. Saudubray and C. Debauche for referring patients 5 and 6 to us. We thank M. Glerum for her gifts of pMGL3, a YEp351 shuttle plasmid with the ADH1 promoter and terminator, and pBCS1H, a cDNA clone containing human BCS1L on a 1.5-kb BamHI fragment. We thank C. Dodé, who provided us the DNA of control individuals from Turkish origin. This research was supported in part by the Association Française contre les Myopathies (7145-AO99), by a research grant from the National Institutes of Health HL22174 (to A.T.) and by grant MDACU01991001 from the Muscular Dystrophy Association (to A.B.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
de Lonlay, P., Valnot, I., Barrientos, A. et al. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat Genet 29, 57–60 (2001). https://doi.org/10.1038/ng706
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng706
This article is cited by
-
Characterization of terminal-ileal and colonic Crohn’s disease in treatment-naïve paediatric patients based on transcriptomic profile using logistic regression
Journal of Translational Medicine (2021)
-
Exploiting pyocyanin to treat mitochondrial disease due to respiratory complex III dysfunction
Nature Communications (2021)
-
A spontaneous mitonuclear epistasis converging on Rieske Fe-S protein exacerbates complex III deficiency in mice
Nature Communications (2020)
-
Mitochondrial UQCRB as a new molecular prognostic biomarker of human colorectal cancer
Experimental & Molecular Medicine (2017)
-
MELAS syndrome and cardiomyopathy: linking mitochondrial function to heart failure pathogenesis
Heart Failure Reviews (2016)