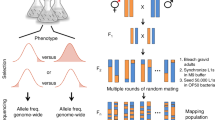
Abstract
Individual response to small-molecule drugs is variable; a drug that provides a cure for some may confer no therapeutic benefit or trigger an adverse reaction in others. To begin to understand such differences systematically, we treated 104 genotyped segregants from a cross between two yeast strains with a collection of 100 diverse small molecules. We used linkage analysis to identify 124 distinct linkages between genetic markers and response to 83 compounds. The linked markers clustered at eight genomic locations, or quantitative-trait locus 'hotspots', that contain one or more polymorphisms that affect response to multiple small molecules. We also experimentally verified that a deficiency in leucine biosynthesis caused by a deletion of LEU2 underlies sensitivity to niguldipine, which is structurally related to therapeutic calcium channel blockers, and that a natural coding-region polymorphism in the inorganic phosphate transporter PHO84 underlies sensitivity to two polychlorinated phenols that uncouple oxidative phosphorylation. Our results provide a step toward a systematic understanding of small-molecule drug action in genetically distinct individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weinstein, J.N. et al. An information-intensive approach to the molecular pharmacology of cancer. Science 275, 343–349 (1997).
Dolan, M.E. et al. Heritability and linkage analysis of sensitivity to cisplatin-induced cytotoxicity. Cancer Res. 64, 4353–4356 (2004).
Watters, J.W., Kraja, A., Meucci, M.A., Province, M.A. & McLeod, H.L. Genome-wide discovery of loci influencing chemotherapy cytotoxicity. Proc. Natl. Acad. Sci. USA 101, 11809–11814 (2004).
Shukla, S.J. & Dolan, M.E. Use of CEPH and non-CEPH lymphoblast cell lines in pharmacogenetic studies. Pharmacogenomics 6, 303–310 (2005).
Le Morvan, V. et al. Relationships between genetic polymorphisms and anticancer drug cytotoxicity vis-à-vis the NCI-60 panel. Pharmacogenomics 7, 843–852 (2006).
Moisan, F., Longy, M., Robert, J. & Le Morvan, V. Identification of gene polymorphisms of human DNA topoisomerase I in the National Cancer Institute panel of human tumour cell lines. Br. J. Cancer 95, 906–913 (2006).
Yarosh, D.B., Pena, A. & Brown, D.A. DNA repair gene polymorphisms affect cytotoxicity in the National Cancer Institute Human Tumour Cell Line Screening Panel. Biomarkers 10, 188–202 (2005).
Stoehlmacher, J. et al. A multivariate analysis of genomic polymorphisms: prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br. J. Cancer 91, 344–354 (2004).
Hohmann, S. The Yeast Systems Biology Network: mating communities. Curr. Opin. Biotechnol. 16, 356–360 (2005).
Foury, F. Human genetic disease: a cross-talk between man and yeast. Gene 195, 1–10 (1997).
Steinmetz, L.M. et al. Systematic screen for human disease genes in yeast. Nat. Genet. 31, 400–404 (2002).
Outeiro, T.F. & Giorgini, F. Yeast as a drug discovery platform in Huntington's and Parkinson's disease. Biotechnol. J. 1, 258–269 (2006).
Ooi, S.L. et al. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet. 22, 56–63 (2006).
Sinha, H., Nicholson, B.P., Steinmetz, L.M. & McCusker, J.H. Complex genetic interactions in a quantitative trait locus. PloS Genet. 2, e13 (2006).
Steinmetz, L.M. et al. Dissecting the architecture of a quantitative trait locus in yeast. Nature 416, 326–330 (2002).
Deutschbauer, A.M. & Davis, R.W. Quantitative trait loci mapped to single-nucleotide resolution in yeast. Nat. Genet. 37, 1333–1340 (2005).
Ben-Ari, G., Zenvirth, D., Sherman, A. & Klutstein, D.L. Four linked genes participate in controlling sporulation efficiency in budding yeast. PLoS Genet. (in the press) (2006).
Brem, R.B., Yvert, G., Clinton, R. & Kruglyak, L. Genetic dissection of transcriptional regulation in budding yeast. Science 296, 752–755 (2002).
Yvert, G. et al. Trans-acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nat. Genet. 35, 57–64 (2003).
Morley, M. et al. Genetic analysis of genome-wide variation in human gene expression. Nature 430, 743–747 (2004).
Monks, S.A. et al. Genetic inheritance of gene expression in human cell lines. Am. J. Hum. Genet. 75, 1094–1105 (2004).
Perlstein, E.O. et al. Revealing complex traits with small molecules and naturally recombinant yeast strains. Chem. Biol. 13, 319–327 (2006).
Brem, R.B. & Kruglyak, L. The landscape of genetic complexity across 5,700 gene expression traits in yeast. Proc. Natl. Acad. Sci. USA 102, 1572–1577 (2005).
Ruderfer, D.M., Pratt, S.C., Seidel, H.S. & Kruglyak, L. Population genomc analysis of outcrossing and recombination in yeast. Nat. Genet. 38, 1077–1081 (2006).
Maro, B., Marty, M.C. & Bornens, M. In vivo and in vitro effects of the mitochondrial uncoupler FCCP on microtubules. EMBO J. 1, 1347–1352 (1982).
Fogel, S., Welch, J.W. & Maloney, D.H. The molecular genetics of copper resistance in Saccharomyces cerevisiae – a paradigm for non-conventional yeasts. J. Basic Microbiol. 28, 147–160 (1988).
Tamura, K. et al. A hap1 mutation in a laboratory strain of Saccharomyces cerevisiae results in decreased expression of ergosterol-related genes and cellular ergosterol content compared to sake yeast. J. Biosci. Bioeng. 98, 159–166 (2004).
Rogers, B. et al. The pleiotropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 3, 207–214 (2001).
Tenreiro, S. et al. AQR1 gene (ORF YNL065w) encodes a plasma membrane transporter of the major facilitator superfamily that confers resistance to short-chain monocarboxylic acids and quinidine in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 292, 741–748 (2002).
Bolster, D.R., Vary, T.C., Kimball, S.R. & Jefferson, L.S. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J. Nutr. 134, 1704–1710 (2004).
Weinbach, E.C. Biochemical basis for the toxicity of pentachlorophenol. Proc. Natl. Acad. Sci. USA 43, 393–397 (1957).
Somerville, L. The metabolism of fungicides. Xenobiotica 16, 1017–1030 (1986).
Bun-Ya, M., Nishimura, M., Harashima, S. & Oshima, Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 11, 3229–3238 (1991).
Thomas, M.R. & O'Shea, E.K. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc. Natl. Acad. Sci. USA 102, 9565–9570 (2005).
Cordes, F.S., Bright, J.N. & Sansom, M.S. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 323, 951–960 (2002).
Yeh, P., Tschumi, A.I. & Kishony, R. Functional classification of drugs by properties of their pairwise interactions. Nat. Genet. 38, 489–494 (2006).
Borissy, A.A. et al. Systematic discovery of multicomponent therapeutics. Proc. Natl. Acad. Sci. USA 100, 7977–7982 (2003).
Staunton, J.E. et al. Chemosensitivity prediction by transcriptional profiling. Proc. Natl. Acad. Sci. USA 98, 10787–10792 (2001).
Marton, M.J., Vazquez de Aldana, C.R., Qui, H., Chakraburtty, K. & Hinnebusch, A.G. Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIFalpha kinase GCN2. Mol. Cell. Biol. 17, 4474–4489 (1997).
Coller, J. & Parker, R. General translational repression by activators of mRNA decapping. Cell 122, 875–886 (2005).
Zhou, X.F., Yang, X., Wang, Q., Coburn, R.A. & Morris, M.E. Effects of dihydropyridines and pyridines on multidrug resistance mediated by breast cancer resistance protein: in vitro and in vivo studies. Drug Metab. Dispos. 33, 1220–1228 (2005).
Urban, T.J. et al. Functional genomics of membrane transporters in human populations. Genome Res. 16, 223–230 (2006).
Jensen, L.T., Ajua-Alemanji, M. & Culotta, V.C. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 278, 42036–42040 (2003).
Broman, K.W., Wu, H., Sen, S. & Churchill, G.A. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19, 889–890 (2003).
Storici, F., Durham, C.L., Gordenin, D.A. & Resnick, M.A. Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast. Proc. Natl. Acad. Sci. USA 100, 14994–14999 (2003).
Acknowledgements
E.O.P. acknowledges F. Storici for technical advice and D. Altshuler for useful discussions. D.C.R acknowledges discussions with R. Schapire, S. Kulkarni and W. Schoendorf. RM11-1a (MATa leu2Δ ura3Δ), RM11-1b (MATα lys2Δ ura3Δ) and BY4716 (MATα lys2Δ) were gifts of B. Garvik (Fred Hutchison Cancer Research Center). This work was supported by the US National Institute of General Medicine Sciences (S.L.S.) and the US National Institute of Mental Health (L.K.). Work at Princeton was supported in part by a Center grant P50GM071508 from the US National Institute of General Medical Science/US National Institutes of Health. L.K. is a James S. McDonnell Centennial Fellow. S.L.S. is an Investigator at the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Complete clustergram. (PDF 907 kb)
Supplementary Table 1
Complete list of SMPs. (PDF 33 kb)
Supplementary Table 2
Raw data. (XLS 620 kb)
Supplementary Table 3
Complete list of SMP/linkages. (PDF 73 kb)
Supplementary Table 4
Collapsed list of SMP/linkages. (PDF 48 kb)
Supplementary Table 5
Collapsed list of confidence intervals. (PDF 64 kb)
Supplementary Table 6
Complete list of QTL hotspots. (PDF 19 kb)
Supplementary Table 7
Primer sequences. (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Perlstein, E., Ruderfer, D., Roberts, D. et al. Genetic basis of individual differences in the response to small-molecule drugs in yeast. Nat Genet 39, 496–502 (2007). https://doi.org/10.1038/ng1991
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1991
This article is cited by
-
The fitness trade-off between growth and stress resistance determines the phenotypic landscape
BMC Biology (2024)
-
Accounting for genetic interactions improves modeling of individual quantitative trait phenotypes in yeast
Nature Genetics (2017)
-
Powerful decomposition of complex traits in a diploid model
Nature Communications (2016)
-
Integrating transcriptional and protein interaction networks to prioritize condition-specific master regulators
BMC Systems Biology (2015)
-
Functional genomics to uncover drug mechanism of action
Nature Chemical Biology (2015)