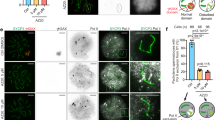
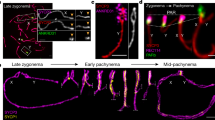
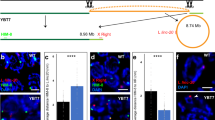
Abstract
In mammalian males, the first meiotic prophase is characterized by formation of a separate chromatin domain called the sex body1. In this domain, the X and Y chromosomes are partially synapsed and transcriptionally silenced, a process termed meiotic sex-chromosome inactivation (MSCI)2,3. Likewise, unsynapsed autosomal chromatin present during pachytene is also silenced (meiotic silencing of unsynapsed chromatin, MSUC)2,4,5. Although it is known that MSCI and MSUC are both dependent on histone H2A.X phosphorylation mediated by the kinase ATR, and cause repressive H3 Lys9 dimethylation4, the mechanisms underlying silencing are largely unidentified. Here, we demonstrate an extensive replacement of nucleosomes within unsynapsed chromatin, depending on and initiated shortly after induction of MSCI and MSUC. Nucleosomal eviction results in the exclusive incorporation of the H3.3 variant, which to date has primarily been associated with transcriptional activity. Nucleosomal exchange causes loss and subsequent selective reacquisition of specific histone modifications. This process therefore provides a means for epigenetic reprogramming of sex chromatin presumably required for gene silencing in the male mammalian germ line.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Handel, M.A. The XY body: a specialized meiotic chromatin domain. Exp. Cell Res. 296, 57–63 (2004).
Turner, J.M. et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37, 41–47 (2005).
Kierszenbaum, A.L. & Tres, L.L. Nucleolar and perichromosomal RNA synthesis during meiotic prophase in the mouse testis. J. Cell Biol. 60, 39–53 (1974).
Turner, J.M., Mahadevaiah, S.K., Ellis, P.J., Mitchell, M.J. & Burgoyne, P.S. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev. Cell 10, 521–529 (2006).
Baarends, W.M. et al. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol. Cell. Biol. 25, 1041–1053 (2005).
Nightingale, K.P., O'neill, L.P. & Turner, B.M. Histone modifications: signalling receptors and potential elements of a heritable epigenetic code. Curr. Opin. Genet. Dev. 16, 125–136 (2006).
Kamakaka, R.T. & Biggins, S. Histone variants: deviants? Genes Dev. 19, 295–310 (2005).
Henikoff, S. & Ahmad, K. Assembly of variant histones into chromatin. Annu. Rev. Cell Dev. Biol. 21, 133–153 (2005).
van der Heijden, G.W. et al. Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech. Dev. 122, 1008–1022 (2005).
Loppin, B. et al. The histone H3.3 chaperone HIRA is essential for chromatin assembly in the male pronucleus. Nature 437, 1386–1390 (2005).
Khalil, A.M., Boyar, F.Z. & Driscoll, D.J. Dynamic histone modifications mark sex chromosome inactivation and reactivation during mammalian spermatogenesis. Proc. Natl. Acad. Sci. USA 101, 16583–16587 (2004).
Namekawa, S.H. et al. Postmeiotic sex chromatin in the male germline of mice. Curr. Biol. 16, 660–667 (2006).
Greaves, I.K., Rangasamy, D., Devoy, M., Marshall Graves, J.A. & Tremethick, D.J. The X and Y chromosomes assemble into H2A.Z, containing facultative heterochromatin, following meiosis. Mol. Cell. Biol. 26, 5394–5405 (2006).
Kramers, K. et al. Specificity of monoclonal anti-nucleosome auto-antibodies derived from lupus mice. J. Autoimmun. 9, 723–729 (1996).
Dietrich, A.J.J. & de Boer, P. A sequential analysis of the development of the synaptonemal complex in spermatocytes of the mouse by electron microscopy using hydroxyurea and agar filtration. Genetica 61, 119–129 (1983).
Tagami, H., Ray-Gallet, D., Almouzni, G. & Nakatani, Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61 (2004).
Hake, S.B. et al. Serine 31 phosphorylation of histone variant H3.3 is specific to regions bordering centromeres in metaphase chromosomes. Proc. Natl. Acad. Sci. USA 102, 6344–6349 (2005).
Mahadevaiah, S.K. et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 27, 271–276 (2001).
Moens, P.B. Histones H1 and H4 of surface-spread meiotic chromosomes. Chromosoma 104, 169–174 (1995).
Oakberg, E.F. Duration of spermatogenesis in the mouse and timing of stages of the cycle in the seminiferous epithelium. Am. J. Anat. 99, 507–516 (1956).
de Boer, P., Searle, A.G., van der Hoeven, F.A., de Rooij, D.G. & Beechey, C.V. Male pachytene pairing in single and double translocation heterozygotes and spermatogenic impairment in the mouse. Chromosoma 93, 326–336 (1986).
Speed, R.M. Abnormal RNA synthesis in sex vesicles of tertiary trisomic male mice. Chromosoma 93, 267–270 (1986).
Turner, J.M. et al. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 14, 2135–2142 (2004).
Baart, E.B., de Rooij, D.G., Keegan, K.S. & de Boer, P. Distribution of Atr protein in primary spermatocytes of a mouse chromosomal mutant: a comparison of preparation techniques. Chromosoma 109, 139–147 (2000).
de Vries, F.A. et al. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 19, 1376–1389 (2005).
van Attikum, H. & Gasser, S.M. The histone code at DNA breaks: a guide to repair? Nat. Rev. Mol. Cell Biol. 6, 757–765 (2005).
Fernandez-Capetillo, O. et al. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev. Cell 4, 497–508 (2003).
Peters, A.H., Plug, A.W., van Vugt, M.J. & de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5, 66–68 (1997).
Ashley, T., Gaeth, A.P., Creemers, L.B., Hack, A.M. & de Rooij, D.G. Correlation of meiotic events in testis sections and microspreads of mouse spermatocytes relative to the mid-pachytene checkpoint. Chromosoma 113, 126–136 (2004).
Kourmouli, N. et al. Heterochromatin and tri-methylated lysine 20 of histone H4 in animals. J. Cell Sci. 117, 2491–2501 (2004).
Acknowledgements
We thank T. Jenuwein, A. Schulmeister, F. van Leeuwen, C. Heyting, P.B. Moens, P.D. Adams and H.G. Stunnenberg for providing antibody reagents; J.-F. Spetz, A. Kelly and M. Puschendorf for their help in the generation and initial characterization of H3.1-HA and H3.3-V5 transgenic mice; C. Heyting, A. Pastink and E. de Boer for male meiotic preparations of Sycp1−/− knockout mice; and W.M. Baarends, C. Logie and P.J. Wang for critical reading of the manuscript. Research in the laboratory of A.H.F.M.P. is supported by the Novartis Research Foundation and the NoE network “The Epigenome” (LSHG-CT-2004-503433).
Author information
Authors and Affiliations
Contributions
G.W.v.d.H., A.A.H.A.D., E.P., A.H.F.M.P. and P.d.B. conceived and designed the experiments. G.W.v.d.H., A.A.H.A.D., E.P. and M.G. performed the experiments. G.W.v.d.H., A.A.H.A.D., E.P., A.H.F.M.P. and P.d.B. analyzed the data. P.P. generated histone-tagged transgenic mice. L.R. was responsible for human material. J.v.d.V. contributed H3.1- and H3.2-specific antibodies. G.W.v.d.H., A.A.H.A.D., D.G.W., J.v.d.V., A.H.F.M.P. and P.d.B. contributed to the writing of the manuscript. J.V.d.V. and A.H.F.M.P. contributed equally to this work.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Temporary reduction of nucleosome density in the XY body. (PDF 828 kb)
Supplementary Fig. 2
Generation and characterization of H3.3-V5 and H3.1-HA transgenic lines. (PDF 157 kb)
Supplementary Fig. 3
Patterns of H3K4, H3K9, H3K27 and H4K20 methylation during prophase I. (PDF 2158 kb)
Supplementary Fig. 4
Schematic overview of timing of events. (PDF 390 kb)
Supplementary Table 1
Presence of HirA in XY chromatin during prophase I. (PDF 23 kb)
Supplementary Table 2
Duration of H3.1/H3.2 loss and positioning in prophase I. (PDF 30 kb)
Supplementary Table 3
Frequencies of H3.3-V5 incorporation into sex chromosomes during subsequent stages of meiotic prophase and in round spermatids. (PDF 34 kb)
Supplementary Table 4
Histone H3.1/H3.2 in T70H/T1Wa and Sycp−/− spermatocytes. (PDF 25 kb)
Rights and permissions
About this article
Cite this article
van der Heijden, G., Derijck, A., Pósfai, E. et al. Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet 39, 251–258 (2007). https://doi.org/10.1038/ng1949
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1949
This article is cited by
-
Active DNA damage response signaling initiates and maintains meiotic sex chromosome inactivation
Nature Communications (2022)
-
Meiotic sex chromosome inactivation and the XY body: a phase separation hypothesis
Cellular and Molecular Life Sciences (2022)
-
A novel posttranslational modification of histone, H3 S-sulfhydration, is down-regulated in asthenozoospermic sperm
Journal of Assisted Reproduction and Genetics (2021)
-
In the line-up: deleted genes associated with DiGeorge/22q11.2 deletion syndrome: are they all suspects?
Journal of Neurodevelopmental Disorders (2019)
-
TH2BS11ph histone mark is enriched in the unsynapsed axes of the XY body and predominantly associates with H3K4me3-containing genomic regions in mammalian spermatocytes
Epigenetics & Chromatin (2019)