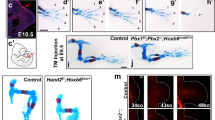
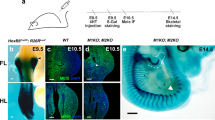
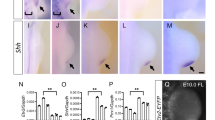
Abstract
Human mutations in TBX5, a gene encoding a T-box transcription factor, and SALL4, a gene encoding a zinc-finger transcription factor, cause similar upper limb and heart defects. Here we show that Tbx5 regulates Sall4 expression in the developing mouse forelimb and heart; mice heterozygous for a gene trap allele of Sall4 show limb and heart defects that model human disease. Tbx5 and Sall4 interact both positively and negatively to finely regulate patterning and morphogenesis of the anterior forelimb and heart. Thus, a positive and negative feed-forward circuit between Tbx5 and Sall4 ensures precise patterning of embryonic limb and heart and provides a unifying mechanism for heart/hand syndromes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Seidman, J.G. & Seidman, C. Transcription factor haploinsufficiency: when half a loaf is not enough. J. Clin. Invest. 109, 451–455 (2002).
Bruneau, B.G. Transcriptional regulation of vertebrate cardiac morphogenesis. Circ. Res. 90, 509–519 (2002).
Garg, V. et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424, 443–447 (2003).
Bruneau, B.G. et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106, 709–721 (2001).
Hiroi, Y. et al. Tbx5 associates with Nkx2–5 and synergistically promotes cardiomyocyte differentiation. Nat. Genet. 28, 276–280 (2001).
Mori, A.D. & Bruneau, B.G. TBX5 mutations and congenital heart disease: Holt-Oram syndrome revealed. Curr. Opin. Cardiol. 19, 211–215 (2004).
Poznanski, A.K., Gall, J.C. Jr . & Stern, A.M. Skeletal manifestations of the Holt-Oram syndrome. Radiology 94, 45–53 (1970).
Basson, C.T. et al. The clinical and genetic spectrum of the Holt-Oram syndrome (heart-hand syndrome). N. Engl. J. Med. 330, 885–891 (1994).
Newbury-Ecob, R.A., Leanage, R., Raeburn, J.A. & Young, I.D. Holt-Oram syndrome: a clinical genetic study. J. Med. Genet. 33, 300–307 (1996).
Tickle, C. Patterning systems–from one end of the limb to the other. Dev. Cell 4, 449–458 (2003).
Litingtung, Y., Dahn, R.D., Li, Y., Fallon, J.F. & Chiang, C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418, 979–983 (2002).
te Welscher, P. et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 298, 827–830 (2002).
Drossopoulou, G. et al. A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signalling and Bmp signalling. Development 127, 1337–1348 (2000).
Dahn, R.D. & Fallon, J.F. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science 289, 438–441 (2000).
Suzuki, T., Takeuchi, J., Koshiba-Takeuchi, K. & Ogura, T. Tbx genes specify posterior digit identity through Shh and BMP signaling. Dev. Cell 6, 43–53 (2004).
Chiang, C. et al. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev. Biol. 236, 421–435 (2001).
Ahn, S. & Joyner, A.L. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505–516 (2004).
Harfe, B.D. et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528 (2004).
Bamshad, M. et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat. Genet. 16, 311–315 (1997).
Davenport, T.G., Jerome-Majewska, L.A. & Papaioannou, V.E. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development 130, 2263–2273 (2003).
Kohlhase, J. et al. Okihiro syndrome is caused by SALL4 mutations. Hum. Mol. Genet. 11, 2979–2987 (2002).
Al-Baradie, R. et al. Duane Radial Ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am. J. Hum. Genet. 71, 1195–1199 (2002).
Kohlhase, J. et al. Mutations at the SALL4 locus on chromosome 20 result in a range of clinically overlapping phenotypes, including Okihiro syndrome, Holt-Oram syndrome, acro-renal-ocular syndrome, and patients previously reported to represent thalidomide embryopathy. J. Med. Genet. 40, 473–478 (2003).
Borozdin, W. et al. Novel mutations in the gene SALL4 provide further evidence for acro-renal-ocular and Okihiro syndromes being allelic entities, and extend the phenotypic spectrum. J. Med. Genet. 41, e102 (2004).
Brassington, A.M. et al. Expressivity of Holt-Oram syndrome is not predicted by TBX5 genotype. Am. J. Hum. Genet. 73, 74–85 (2003).
Kohlhase, J. et al. Cloning and expression analysis of SALL4, the murine homologue of the gene mutated in Okihiro syndrome. Cytogenet. Genome Res. 98, 274–277 (2002).
Spitz, F., Gonzalez, F. & Duboule, D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 113, 405–417 (2003).
Pizard, A. et al. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits and sternum. Mol. Cell. Biol. 25, 5073–5083 (2005).
Sekine, K. et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 21, 138–141 (1999).
Naiche, L.A. & Papaioannou, V.E. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 130, 2681–2693 (2003).
Szeto, D.P. et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 13, 484–494 (1999).
Logan, M. & Tabin, C.J. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science 283, 1736–1739 (1999).
Lanctot, C., Moreau, A., Chamberland, M., Tremblay, M.L. & Drouin, J. Hindlimb patterning and mandible development require the Ptx1 gene. Development 126, 1805–1810 (1999).
Agarwal, P. et al. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development 130, 623–633 (2003).
Gibson-Brown, J.J. et al. Evidence of a role for T-box genes in the evolution of limb morphogenesis and the specification of forelimb/hindlimb identity. Mech. Dev. 56, 93–101 (1996).
Logan, M. Finger or toe: the molecular basis of limb identity. Development 130, 6401–6410 (2003).
Habets, P.E. et al. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 16, 1234–1246 (2002).
Hoogaars, W.M. et al. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc. Res. 62, 489–499 (2004).
Ghosh, T.K. et al. Characterization of the TBX5 binding site and analysis of mutations that cause Holt-Oram syndrome. Hum. Mol. Genet. 10, 1983–1994 (2001).
Fan, C., Liu, M. & Wang, Q. Functional analysis of TBX5 missense mutations associated with Holt-Oram syndrome. J. Biol. Chem. 278, 8780–8785 (2003).
Durocher, D., Charron, F., Warren, R., Schwartz, R.J. & Nemer, M. The cardiac transcription factors Nkx2–5 and GATA-4 are mutual cofactors. EMBO J. 16, 5687–5696 (1997).
Minguillon, C., Del Buono, J. & Logan, M.P. Tbx5 and Tbx4 are not sufficient to determine limb-specific morphologies but have common roles in initiating limb outgrowth. Dev. Cell 8, 75–84 (2005).
Rallis, C. et al. Tbx5 is required for forelimb bud formation and continued outgrowth. Development 130, 2741–2751 (2003).
Shen-Orr, S.S., Milo, R., Mangan, S. & Alon, U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 31, 64–68 (2002).
Mangan, S. & Alon, U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA 100, 11980–11985 (2003).
Boyer, L.A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Kaufman, M.H. The Atlas of Mouse Development (Academic, London, 1992).
Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432, 107–112 (2004).
Acknowledgements
We are grateful to A. Mori for help with statistics and quantitative RT-PCR. We also thank V. Christoffels, M. Nemer and T. Ogura for expression vectors and reporter constructs, and A. Brown, D. Duboule, M. Logan, G. Martin and C. Oka for in situ probes. This work was supported by grants from the Canadian Institutes of Health Research (B.G.B., C.c.H), the Heart and Stroke Foundation of Ontario (B.G.B.) and the March of Dimes Birth Defects Foundation (B.G.B.). K.K.-T. was supported by the Uehara Memorial Foundation. J.K.T holds a long-term fellowship from the Human Frontiers Science Program and was partly supported by the Mochida Memorial Foundation for Medical and Pharmaceutical Research. B.G.B. holds a Canada Research Chair in Developmental Cardiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic of Sall4 alleles. (PDF 179 kb)
Supplementary Fig. 2
Patterning defects in Tbx5−/+ limbs. (PDF 425 kb)
Supplementary Fig. 3
Nuclear localization of Sall4 proteins. (PDF 307 kb)
Rights and permissions
About this article
Cite this article
Koshiba-Takeuchi, K., Takeuchi, J., Arruda, E. et al. Cooperative and antagonistic interactions between Sall4 and Tbx5 pattern the mouse limb and heart. Nat Genet 38, 175–183 (2006). https://doi.org/10.1038/ng1707
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1707
This article is cited by
-
SALL4 deletion and kidney and cardiac defects associated with VACTERL association
Pediatric Nephrology (2024)
-
Functional analysis of two novel TBX5 variants present in individuals with Holt–Oram syndrome with different clinical manifestations
Molecular Genetics and Genomics (2021)
-
Structural bases of IMiD selectivity that emerges by 5-hydroxythalidomide
Nature Communications (2020)
-
Identification and functional analysis of genetic variants in TBX5 gene promoter in patients with acute myocardial infarction
BMC Cardiovascular Disorders (2019)
-
The role of ESCO2, SALL4 and TBX5 genes in the susceptibility to thalidomide teratogenesis
Scientific Reports (2019)