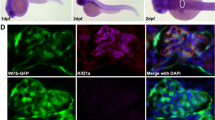
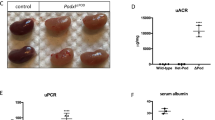
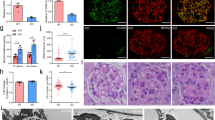
Abstract
Progressive kidney failure is a genetically and clinically heterogeneous group of disorders. Podocyte foot processes and the interposed glomerular slit diaphragm are essential components of the permeability barrier in the kidney. Mutations in genes encoding structural proteins of the podocyte lead to the development of proteinuria, resulting in progressive kidney failure and focal segmental glomerulosclerosis. Here, we show that the canonical transient receptor potential 6 (TRPC6) ion channel is expressed in podocytes and is a component of the glomerular slit diaphragm. We identified five families with autosomal dominant focal segmental glomerulosclerosis in which disease segregated with mutations in the gene TRPC6 on chromosome 11q. Two of the TRPC6 mutants had increased current amplitudes. These data show that TRPC6 channel activity at the slit diaphragm is essential for proper regulation of podocyte structure and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zandi-Nejad, K., Eddy, A.A., Glassock, R.J. & Brenner, B.M. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. Suppl., S76–S89 (2004).
Somlo, S. & Mundel, P. Getting a foothold in nephrotic syndrome. Nat. Genet. 24, 333–335 (2000).
Pavenstadt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Pollak, M.R. Inherited podocytopathies: FSGS and nephrotic syndrome from a genetic viewpoint. J. Am. Soc. Nephrol. 13, 3016–3023 (2002).
Winn, M.P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science, published online 5 May 2005 (10.1126/science.1106215).
Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, re3 (2005).
Clapham, D.E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Facemire, C.S., Mohler, P.J. & Arendshorst, W.J. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am. J. Physiol. Renal Physiol. 286, F546–F551 (2004).
Mundel, P. et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 139, 193–204 (1997).
Mundel, P. et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236, 248–258 (1997).
Schnabel, E., Dekan, G., Miettinen, A. & Farquhar, M.G. Biogenesis of podocalyxin–the major glomerular sialoglycoprotein–in the newborn rat kidney. Eur. J. Cell Biol. 48, 313–326 (1989).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Kim, J.M. et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300, 1298–1300 (2003).
Reiser, J. et al. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J. Clin. Invest. 113, 1390–1397 (2004).
Putaala, H., Soininen, R., Kilpelainen, P., Wartiovaara, J. & Tryggvason, K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum. Mol. Genet. 10, 1–8 (2001).
Hamano, Y. et al. Determinants of vascular permeability in the kidney glomerulus. J. Biol. Chem. 277, 31154–31162 (2002).
Kaplan, J.M. et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Pavenstadt, H. & Bek, M. Podocyte electrophysiology, in vivo and in vitro. Microsc. Res. Tech. 57, 224–227 (2002).
Drenckhahn, D. & Franke, R.P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab. Invest. 59, 673–682 (1988).
Huber, T.B. et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 23, 4917–4928 (2003).
Verma, R. et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J. Biol. Chem. 278, 20716–20723 (2003).
Hisatsune, C. et al. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 279, 18887–18894 (2004).
Bandyopadhyay, B.C. et al. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J. Biol. Chem. 280, 12908–12916 (2005).
Reiser, J., Kriz, W., Kretzler, M. & Mundel, P. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 11, 1–8 (2000).
Notredame, C., Higgins, D.G. & Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 (2000).
Acknowledgements
We thank the family members for their participation in these studies and M.A. Arnaout for discussions about the manuscript. This work was supported by grants from the US National Institutes of Health (M.R.P., P.M. and R.K.) as well as the Howard Hughes Medical Institute (P.L.S. and D.E.C.). J.R. was supported by the KMD Foundation and the KUFA-ASN Research Grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Summary of TRPC6 mutations. (PDF 99 kb)
Supplementary Table 1
Primer sequences. (PDF 43 kb)
Supplementary Note
Clinical information. (PDF 40 kb)
Rights and permissions
About this article
Cite this article
Reiser, J., Polu, K., Möller, C. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37, 739–744 (2005). https://doi.org/10.1038/ng1592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1592
This article is cited by
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases
Signal Transduction and Targeted Therapy (2023)
-
An inactivating human TRPC6 channel mutation without focal segmental glomerulosclerosis
Cellular and Molecular Life Sciences (2023)
-
Hidden genetics behind glomerular scars: an opportunity to understand the heterogeneity of focal segmental glomerulosclerosis?
Pediatric Nephrology (2023)
-
Transient Receptor Potential Canonical 6 (TRPC6) Channel in the Pathogenesis of Diseases: A Jack of Many Trades
Inflammation (2023)
-
Mutations in trpγ, the homologue of TRPC6 autism candidate gene, causes autism-like behavioral deficits in Drosophila
Molecular Psychiatry (2022)