Abstract
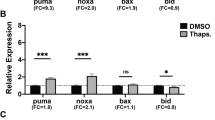
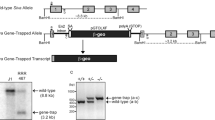
The role of transcriptional activation in p53 function is highly controversial. To define this role in vivo, we generated a Trp53 knock-in construct encoding a protein carrying mutations of two residues that are crucial for transactivation (L25Q,W26S). Here we show that these mutations have selective effects on the biological functions of p53. Although its ability to activate various p53 target genes is largely compromised, the p53QS protein retains the ability to transactivate the gene Bax. The ability of the p53QS mutant protein to elicit a DNA damage–induced G1 cell cycle–arrest response is also partially impaired. p53QS has selective defects in its ability to induce apoptosis: it is completely unable to activate apoptosis in response to DNA damage, is partially unable to do so when subjected to serum deprivation and retains substantial apoptotic activity upon exposure to hypoxia. These findings suggest that p53 acts through distinct, stimulus-specific pathways to induce apoptosis. The importance of the biological activity of p53QS in vivo is underscored by our finding that expression of p53QS, which cannot bind mdm2, induces embryonic lethality. Taken together, these results suggest that p53 has different mechanisms of action depending on specific contextual cues, which may help to clarify the function of p53 in preventing cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Donehower, L.A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994).
el-Deiry, W.S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Brugarolas, J. et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377, 552–557 (1995).
Deng, C., Zhang, P., Harper, J.W., Elledge, S.J. & Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 (1995).
Vousden, K.H. & Lu, X. Live or let die: the cell's response to p53. Nat. Rev. Cancer 2, 594–604 (2002).
Sabbatini, P., Lin, J., Levine, A.J. & White, E. Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev. 9, 2184–2192 (1995).
Attardi, L.D., Lowe, S.W., Brugarolas, J. & Jacks, T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 15, 3702–3712 (1996).
McCurrach, M.E., Connor, T.M., Knudson, C.M., Korsmeyer, S.J. & Lowe, S.W. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94, 2345–2349 (1997).
Ihrie, R.A. et al. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr. Biol. 13, 1985–1990 (2003).
Knudson, C.M., Tung, K.S., Tourtellotte, W.G., Brown, G.A. & Korsmeyer, S.J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270, 96–99 (1995).
Shibue, T. et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 17, 2233–2238 (2003).
Caelles, C., Helmberg, A. & Karin, M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370, 220–223 (1994).
Wagner, A.J., Kokontis, J.M. & Hay, N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 8, 2817–2830 (1994).
Chen, X., Ko, L.J., Jayaraman, L. & Prives, C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10, 2438–2451 (1996).
Haupt, Y., Rowan, S., Shaulian, E., Vousden, K.H. & Oren, M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 9, 2170–2183 (1995).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Dumont, P., Leu, J.I., Della Pietra, A.C. 3rd, George, D.L. & Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 33, 357–365 (2003).
Chipuk, J.E. et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010–1014 (2004).
Lin, J., Chen, J., Elenbaas, B. & Levine, A.J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 8, 1235–1246 (1994).
Uesugi, M. & Verdine, G.L. The alpha-helical FXXPhiPhi motif in p53: TAF interaction and discrimination by MDM2. Proc. Natl. Acad. Sci. USA 96, 14801–14806 (1999).
Montes de Oca Luna, R., Wagner, D.S. & Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203–206 (1995).
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003).
Ludwig, R.L., Bates, S. & Vousden, K.H. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol. Cell. Biol. 16, 4952–4960 (1996).
Friedlander, P., Haupt, Y., Prives, C. & Oren, M. A mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol. Cell. Biol. 16, 4961–4971 (1996).
Kastan, M.B. et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71, 587–597 (1992).
Attardi, L.D., de Vries, A. & Jacks, T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene 23, 973–980 (2004).
Lowe, S.W. & Ruley, H.E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 7, 535–545 (1993).
Soengas, M.S. et al. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284, 156–159 (1999).
Hammond, E.M., Denko, N.C., Dorie, M.J., Abraham, R.T. & Giaccia, A.J. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22, 1834–1843 (2002).
Chao, C. et al. p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J. 19, 4967–4975 (2000).
Zhu, J., Zhou, W., Jiang, J. & Chen, X. Identification of a novel p53 functional domain that is necessary for mediating apoptosis. J. Biol. Chem. 273, 13030–13036 (1998).
Venot, C., Maratrat, M., Sierra, V., Conseiller, E. & Debussche, L. Definition of a p53 transactivation function-deficient mutant and characterization of two independent p53 transactivation subdomains. Oncogene 18, 2405–2410 (1999).
Hemann, M.T. et al. Suppression of tumorigenesis by the p53 target PUMA. Proc. Natl. Acad. Sci. USA 101, 9333–9338 (2004).
Koumenis, C. et al. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol. Cell. Biol. 21, 1297–1310 (2001).
Kokontis, J.M., Wagner, A.J., O'Leary, M., Liao, S. & Hay, N. A transcriptional activation function of p53 is dispensable for and inhibitory of its apoptotic function. Oncogene 20, 659–668 (2001).
Chavez-Reyes, A. et al. Switching mechanisms of cell death in mdm2- and mdm4-null mice by deletion of p53 downstream targets. Cancer Res. 63, 8664–8669 (2003).
Fischer, B. & Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. 99, 673–679 (1993).
Akazawa, S., Unterman, T. & Metzger, B.E. Glucose metabolism in separated embryos and investing membranes during organogenesis in the rat. Metabolism 43, 830–835 (1994).
Adelman, D.M., Gertsenstein, M., Nagy, A., Simon, M.C. & Maltepe, E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 14, 3191–3203 (2000).
Jimenez, G.S. et al. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat. Genet. 26, 37–43 (2000).
Chen, J., Wu, X., Lin, J. & Levine, A.J. mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol. Cell. Biol. 16, 2445–2452 (1996).
Tuveson, D.A. et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Livingstone, L.R. et al. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 70, 923–935 (1992).
Attardi, L.D. et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14, 704–718 (2000).
Adimoolam, S. & Ford, J.M. p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc. Natl. Acad. Sci. USA 99, 12985–12990 (2002).
Reczek, E.E., Flores, E.R., Tsay, A.S., Attardi, L.D. & Jacks, T. Multiple response elements and differential p53 binding control Perp expression during apoptosis. Mol. Cancer Res. 1, 1048–1057 (2003).
Acknowledgements
We thank D. Tuveson for providing the transcriptional stop cassette; T. Jacks for providing the Trp53LSL-LW mice; N. Denko for providing the Slc2a1 probe; R. Freiberg and S. Basak for technical assistance; and S. Artandi, A. Brunet, R. Ihrie and J. Sage for critical reading of the manuscript. This work was supported by a Lucille P. Markey Biomedical Research Stanford Graduate Fellowship and a National Science Foundation Graduate Research Fellowship to T.M.J., by the National Institutes of Health to A.G. and by the Damon Runyon Cancer Research Foundation to L.D.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
UV radiation induces p53-dependent and independent apoptosis. (PDF 47 kb)
Rights and permissions
About this article
Cite this article
Johnson, T., Hammond, E., Giaccia, A. et al. The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat Genet 37, 145–152 (2005). https://doi.org/10.1038/ng1498
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1498
This article is cited by
-
The oncogenicity of tumor-derived mutant p53 is enhanced by the recruitment of PLK3
Nature Communications (2021)
-
Shifting the paradigms for tumor suppression: lessons from the p53 field
Oncogene (2021)
-
Siva plays a critical role in mouse embryonic development
Cell Death & Differentiation (2020)
-
Deconstructing networks of p53-mediated tumor suppression in vivo
Cell Death & Differentiation (2018)
-
BIM and NOXA are mitochondrial effectors of TAF6δ-driven apoptosis
Cell Death & Disease (2018)