Abstract
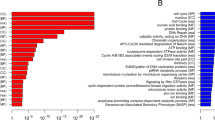
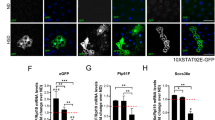
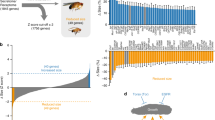
Insulin-IGF receptor (InR) signaling has a conserved role in regulating lifespan, but little is known about the genetic control of declining organ function. Here, we describe progressive changes of heart function in aging fruit flies: from one to seven weeks of a fly's age, the resting heart rate decreases and the rate of stress-induced heart failure increases. These age-related changes are minimized or absent in long-lived flies when systemic levels of insulin-like peptides are reduced and by mutations of the only receptor, InR, or its substrate, chico. Moreover, interfering with InR signaling exclusively in the heart, by overexpressing the phosphatase dPTEN or the forkhead transcription factor dFOXO, prevents the decline in cardiac performance with age. Thus, insulin-IGF signaling influences age-dependent organ physiology and senescence directly and autonomously, in addition to its systemic effect on lifespan. The aging fly heart is a model for studying the genetics of age-sensitive organ-specific pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Guarente, L. & Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262 (2000).
Helfland, S.L. & Rogina, B. Molecular genetics of aging in the fly: is this the end of the beginning? Bioessays 25, 134–141 (2003).
Tatar, M., Bartke, A. & Antebi, A. The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351 (2003).
Herndon, L.A. et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419, 808–814 (2002).
Garigan, D. et al. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 161, 1101–1112 (2002).
Morley, J.F., Brignull, H.R., Weyers, J.J. & Morimoto, R.I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99, 10417–10422 (2002).
Hwangbo, D.S., Gersham, B., Tu, M.P., Palmer, M. & Tatar, M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566 (2004).
Lakatta, E.G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
Paternostro, G. et al. Age-associated cardiac dysfunction in Drosophila melanogaster. Circ. Res. 88, 1053 (2001).
Wessells, R.J. & Bodmer, R. Screening assays for heart function mutants in Drosophila. Biotechniques 37, 58–60 (2004).
Bodmer, R. Heart development in Drosophila and its relationship to vertebrate systems. Trends Cardiovasc. Med. 5, 21–27 (1995).
Dowse, H. et al. A congenital heart defect in Drosophila caused by an action-potential mutation. J. Neurogenetics 10, 153–168 (1995).
Khan, A.S., Sane, D.C., Wannenburg, T. & Sonntag, W.E. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc. Res. 54, 25–35 (2002).
Tu, M.-P., Epstein, D. & Tatar, M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homolog chico. Aging Cell 1, 75–80 (2002).
Promislow, D.E., Tatar, M., Khazaeli, A.A. & Curtzinger, J.W. Age-specific patterns of genetic variance in Drosophila melanogaster. I. Mortality. Genetics 143, 839–848 (1996).
Leevers, S. Growth control: invertebrate insulin surprises! Curr. Biol. 11, R209–R212 (2001).
Stocker, H. & Hafen, E. Genetic control of cell size. Curr. Opin. Genet. Dev. 10, 529–535 (2000).
Garofalo, R.S. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol. Metab. 13, 156–162 (2002).
Tatar, M. et al. A mutant Drosophila insulin receptor homolog that extends lifespan and impairs neuroendocrine function. Science 292, 107–110 (2001).
Clancy, D.J. et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (2001).
Bohni, R. et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875 (1999).
Siegmund, T. & Korge, G. Innervation of the ring gland of Drosophila melanogaster. J. Comp. Neurol. 431, 481–491 (2001).
Rulifson, E.J., Kim, S.K. & Nusse, R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 (2002).
Apfeld, J. & Kenyon, C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809 (1999).
Wolkow, C.A., Kimura, K.D., Lee, M.S. & Ruvkun, G. Regulation of C. elegans lifespan by insulinlike signaling in the nervous system. Science 290, 147–150 (2000).
Gerisch, B., Weitzel, C., Kober-Eizermann, C., Rottiers, V. & Antebi, A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, an life span. Dev. Cell 1, 841–851 (2001).
Tu, M.-P., Yin, C.-M. & Tatar, M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell 1, 158–160 (2002).
Bluher, M., Kahn, B.B. & Kahn, C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574 (2003).
Libina, N., Berman, J.R. & Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115, 489–502 (2003).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Oldham, S. et al. The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP(3) levels. Development 129, 4103–4109 (2002).
Puig, O., Marr, M., Ruhf, M. & Tjian, R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17, 2006–2020 (2003).
Junger, M.A. et al. The Drosophila Forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2, 20 (2003).
Gao, X., Neufeld, T.P. & Pan, D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and –independent pathways. Dev. Biol. 221, 404–418 (2000).
Fernandez, R., Tabarini, D., Azpiazu, N. Frasch, M. & Schlessinger, J. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 17, 3373–3384 (1995).
Chen, C., Jack, J. & Garofalo, R.S. The Drosophila insulin receptor is required for normal growth. Endocrinology 137, 846–856 (1996).
Venkatesh, T.V. et al. Cardiac enhancer activity of the homeobox gene tinman depends on CREB consensus binding sites in Drosophila. Genesis 26, 55–66 (2000).
Bodmer, R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 118, 719–729 (1993).
Molina, M.R. & Cripps, R.M. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 109, 51–59 (2001).
Hassan, B.A. et al. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron 25, 549–561 (2000).
Tatar, M., Priest, N. & Chien, S. Negligible Senescence during Reproductive Dormancy in Drosophila melanogaster. Am. Nat. 158, 248–258 (2001).
Acknowledgements
We thank B. Edgar, E. Hafen, E. Rulifson, B. Hassan, H. Bellen and the Bloomington stock center for sending flies and K. Fitzgerald, A. Strobe and M. Montero for technical assistance with part of the heart performance assays. R.J.W. has been supported by a fellowship from the American Heart Association. This work received support from grants from National Institutes of Health (National Institute on Aging) and The Ellison Medical Foundation to M.T. and by National Institutes of Health (National Heart, Lung and Blood Institute and National Institute on Aging) to R.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Arrest rates. (PDF 117 kb)
Supplementary Table 1
Summary of failure, arrest and recovery data for selected genotypes. (PDF 8 kb)
Supplementary Table 2
Adult heart rate data from relevant genotypes is displayed. (PDF 6 kb)
Supplementary Table 3
Comparison of male and female failure rate data. (PDF 6 kb)
Supplementary Table 4
Failure rates at 1 week and 5 weeks for outcrossed flies carrying UAS expression constructs. (PDF 6 kb)
Supplementary Table 5
Pupal heart rates from several wild-type genetic backgrounds are compared to heart rates of pupae expressing InR, dPTEN or dFOXO in the heart, as well as flies carrying each construct outcrossed in to wild-type genetic backgrounds. (PDF 6 kb)
Supplementary Video 1
GMH5-driven GFP expression in the adult myocardium of the heart in abdominal segments A2-A4. Spiral myofibril structure in the GFP-labeled contractile myocardial cells is evident34, and thus demonstrating the myocardial specificity of GFP expression by the GMH5 driver. (AVI 28715 kb)
Rights and permissions
About this article
Cite this article
Wessells, R., Fitzgerald, E., Cypser, J. et al. Insulin regulation of heart function in aging fruit flies. Nat Genet 36, 1275–1281 (2004). https://doi.org/10.1038/ng1476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1476
This article is cited by
-
Metabolic landscape in cardiac aging: insights into molecular biology and therapeutic implications
Signal Transduction and Targeted Therapy (2023)
-
Non-invasive red-light optogenetic control of Drosophila cardiac function
Communications Biology (2020)
-
Intergenerational inheritance of high fat diet-induced cardiac lipotoxicity in Drosophila
Nature Communications (2019)
-
Regulation of Drosophila Lifespan by bellwether Promoter Alleles
Scientific Reports (2017)
-
Sexual dimorphism in obesity-related genes in the epicardial fat during aging
Journal of Physiology and Biochemistry (2017)