Abstract
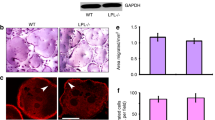
Solubilization of bone mineral by osteoclasts depends on the formation of an acidic extracellular compartment through the action of a V-proton pump that has not yet been characterized at the molecular level1,2,3. We previously cloned a gene (Atp6i, for V-proton pump, H+ transporting (vacuolar proton pump) member I) encoding a putative osteoclast-specific proton pump subunit, termed OC-116kD (ref. 4). Here we show that targeted disruption of Atp6i in mice results in severe osteopetrosis. Atp6i–/– osteoclast-like cells (OCLs) lose the function of extracellular acidification, but retain intracellular lysosomal proton pump activity. The pH in Atp6i–/– liver lysosomes and proton transport in microsomes of Atp6i–/– kidney are identical to that in wild-type mice. Atp6i–/– mice exhibit a normal acid-base balance in blood and urine. Our results demonstrate that Atp6i is unique and necessary for osteoclast-mediated extracellular acidification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vaes, G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J. Cell Biol. 39, 676–697 (1968).
Baron, R., Neff, L., Louvard, D. & Courtoy, P.J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 101, 2210–2222 (1985).
Blair, H.C. & Schlesinger, P.H. in Biology and Physiology of the Osteoclast (eds Rifkin, B.R. & Gay, C.V.) 259–287 (CRC Press, Boca Raton, Florida, 1992).
Li, Y.P., Chen, W. & Stashenko, P. Molecular cloning and characterization of a putative novel human osteoclast-specific 116-kDa vacuolar proton pump subunit. Biochem. Biophys. Res. Commun. 218, 813–821 (1996).
Gerritsen, E.J. et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics 93, 247–253 (1994).
Wang, Z.Q. et al. Bone and haematopoietic defects in mice lacking c-fos. Nature 360, 741–745 (1992).
Tondravi, M.M. et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81–84 (1997).
Iotsova, V. et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nature Med. 3, 1285–1289 (1997).
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Chen, W. & Li, Y.P. Generation of mouse osteoclastogenic cell lines immortalized with SV40 large T antigen. J. Bone Miner. Res. 13, 1112–1123 (1998).
Zimmerli, S. et al. Phagosome-lysosome fusion is a calcium-independent event in macrophages. J. Cell Biol. 132, 49–61 (1996).
Ohkuma, S., Moriyama, Y. & Takano, T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc. Natl Acad. Sci. USA 79, 2758–2762 (1982).
David, P. & Baron, R. The catalytic cycle of the vacuolar H(+)-ATPase. Comparison of proton transport in kidney- and osteoclast-derived vesicles. J. Biol. Chem. 269, 30158–30163 (1994).
Horton, W.A. The biology of bone growth. Growth Genet. Horm. 6, 1–3 (1990).
Johansson, N. et al. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev. Dyn. 208, 387–397 (1997).
Li, E., Bestor, T.H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Laird, P.W. et al. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 19, 4293 (1991).
Tamura, T. et al. New resorption assay with mouse osteoclast-like multinucleated cells formed in vitro. J. Bone Miner. Res. 8, 953–960 (1993).
Shibutani, T. & Heersche, J.N. Effect of medium pH on osteoclast activity and osteoclast formation in cultures of dispersed rabbit osteoclasts. J. Bone Miner. Res. 8, 331–336 (1993).
Li, Y.P. & Chen, W. Characterization of mouse cathepsin K gene, the gene promoter, and the gene expression. J. Bone Miner. Res. 14, 487–499 (1999).
Acknowledgements
We thank D. Hay, S. Orlando and L.J. Maltais for critical reading of the manuscript; W. Deng for assistance with the manuscript; J. Dobeck for histological assistance; and R. Kent and S.-I. A. Liao for statistical analysis assistance. This work was supported by NIH grants DE-07378 (P.S.) and AR44741 (Y.-P.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YP., Chen, W., Liang, Y. et al. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet 23, 447–451 (1999). https://doi.org/10.1038/70563
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/70563