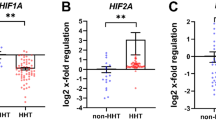
Abstract
Hereditary haemorrhagic telangiectasia (HHT) is an autosomal dominant disorder characterized by multisystemic vascular dysplasia and recurrent haemorrhage. Linkage for some families has been established to chromosome 9q33–q34. In the present study, endoglin, a transforming growth factor β (TGF-β) binding protein, was analysed as a candidate gene for the disorder based on chromosomal location, expression pattern and function. We have identified mutations in three affected individuals: a C to G substitution converting a tyrosine to a termination codon, a 39 base pair deletion and a 2 base pair deletion which creates a premature termination codon. We have identified endoglin as the HHT gene mapping to 9q3 and have established HHT as the first human disease defined by a mutation in a member of the TGF-β receptor complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jahnke, V. Ultrastructure of hereditary telangiectasia. Arch. Otolaryngol. 91, 262–265 (1970).
Hashimoto, K. & Pritzker, M. Hereditary hemorrhagic telangiectasia: an electron microscope study. Oral Surg., Oral Med., Oral Pathol. 34, 751–768 (1972).
Braverman, I.M., Keh, A. & Jacobson, B.S. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J. invest. Dermatol. 95, 422–427 (1990).
Menefee, M.G., Flessa, H.C., Glueck, H.I. & Hogg, S.P. Hereditary hemorrhagic telangiectasia: (Osler-Weber-Rendu disease): an electron microscopic study of the vascular lesions before and after therapy with hormones. Arch. Otolaryngol. 101, 246–251 (1975).
McDonald, M.T. et al. A disease locus for hereditary haemorrhagic telangiectasia maps to chromosome 9q33–34. Nature Genet. 6, 197–204 (1994).
Shovlin, C. et al. A gene for hereditary haemorrhagic telangiectasia maps to chromosome 9q3. Nature Genet. 6, 205–209 (1994).
McAllister, K.A. et al. Genetic heterogeneity in hereditary hemorrhagic telangiectasia: possible correlation with clinical phenotype. J. med. Genet. 31, 927–932 (1994).
Porteous, M.E.M. et al. Genetic heterogeneity in hereditary haemorrhagic telangiectasia. J. med. Genet. 31, 925–926 (1994).
Heutink, P. et al. Linkage of hereditary hemorrhagic telangiectasia to chromosome 9q34 and evidence for locus heterogeneity. J. med. Genet. 31, 933–936 (1994).
Greenspan, D.S. et al. COL5A1: Fine genetic mapping and exclusion as candidate gene in families with nail-patella syndrome, tiberous sclerosis 1, hereditary hemorrhagic telangiectasia and Ehlers-Danlos syndrome type II. Genomics (in the press).
Gougos, A. & Letarte, M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J. biol. Chem. 265, 8361–8364 (1990).
Cheifetz, S. et al. Endoglin is a component of the transforming growth factor-b receptor system in human endothelial cells. J. biol. Chem. 267, 19027–19030 (1992).
Yamashita, H. et al. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-β. J. biol. Chem. 269, 1995–2001 (1994).
Massagué, J., Attisano, L. & Wrana, J.L. The TGF-β family and its composite receptors. Trends cell Biol. 4, 172–178 (1994).
Fernández-Ruiz, E., St-Jacques, S., Bellón, T., Letarte, M. & Bernabeu, C. Assignment of the human endoglin gene (END) to 9q34–qter. Cytogenet. Cell Genet. 64, 204–207 (1993).
Pilz, A., Woodward, K., Peters, J., Povey, S & Abbot, C. Comparative mapping of 38 human chromosome 9 loci in the laboratory mouse. Abstract from the third international workshop on chromosome 9. Ann. hum. Genet. 58, 231–232 (1994).
Povey, S. et al. Report on the third international workshop on human chromosome 9. Ann. hum. Genet. 58, 177–199 (1994).
Leversha, M.A., Carter, N.P. & Ferguson-Smith, M.A. Physical mapping in 9q using fluorescence in situhybridization. Abstract from the third international workshop on chromosome 9. Ann. hum. Genet. 58, 224 (1994).
Lastres, P. et al. Regulated expression on human macrophages of endoglin, an RGD containing surface antigen. Eur.J.Immunol. 22, 393–397 (1992).
Gougos, A. et al. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int. Immunol. 4, 83–92 (1992).
St-Jacques, S., Cymerman, U., Pece, N. & Letarte, M. Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-β binding protein of endothelial and stromal cells. Endocrinology 134, 2645–2657 (1994).
Madri, J.A. et al. Interactions of matrix components and soluble factors in vascular responses to injury. Modulation of cell phenotype. in Endothelial cell dysfunctions (eds Simionescu, N. & Simionescu, M.) (Plenum Press, New York, (1992).
Jennings, J.C., Mohan, S., Linkhart, T.A., Widstrom, R. & Baylink, D.J. Comparison of the biological actions of TGF beta-1 and TGF beta-2: differential activity in endotheiial cells. J. cell. Physiol. 137, 167–172 (1988).
Luscinkas, F.W. & Lawler, J. Integrins as dynamics regulators of vascular function. FASEB J. 8, 929–938 (1994).
Wrana, J.L. et al. TGF-β signals through a heteromeric protein kinase receptor complex. Cell 71, 1003–1014 (1992).
Wrana, J.L., Attisano, A., Wieser, R., Ventura, F., Massagué, J. Mechanisms of activation of the TGF-β receptor. Nature 370, 341–347 (1994).
López-Casillas, F. et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-β receptor. Cell 67, 785–795 (1991).
Wang, X.-F. et al. Expression cloning and characterization of the TGF-β type III receptor. Cell 67, 797–805 (1991).
López-Casillas, F., Wrana, J.L. & Massagué, J. Betaglycan presents ligand to the TGF-β signaling receptor. Cell 73, 1435–1444 (1993).
Cheifetz, S. et al. Distinct transforming growth factor-β (TGF-β) receptor subsets as determinants of cellular responsiveness to three TGF-β isoforms. J. biol. Chem. 265, 20533–20538 (1990).
Shull, M.M. et al. Targeted disruption of the mouse transforming growth factor-β 1 gene results in multifocal inflammatory disease. Nature 359, 693–699 (1992).
Kulkarni, A.B. et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc. natn. Acad. Sci. U.S.A. 90, 770–774 (1993).
Letterio, J.J. et al. Maternal rescue of transforming growth factor-β1 null mice. Science 264, 1936–1938 (1994).
Mathew, S. et al. Transforming growth factor gene TGFBR2 maps to human chromosome band 3p22. Genomics 20, 114–115 (1994).
Blanchard, M.M., Taillon-Miller, P., Nowotny, P. & Nowotny, V. PCR buffer optimization with uniform temperature regimen to facilitate automation. PCR Meth. Applic. 2, 234–240 (1993).
Bellón, T. et al. Identification and expression of two forms of the human transforming growth-factor-β binding-protein endoglin with distinct cytoplasmic regions. Eur. J. Immunol. 23, 2340–2345 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McAllister, K., Grogg, K., Johnson, D. et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8, 345–351 (1994). https://doi.org/10.1038/ng1294-345
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1294-345
This article is cited by
-
NRP1 interacts with endoglin and VEGFR2 to modulate VEGF signaling and endothelial cell sprouting
Communications Biology (2024)
-
Genetics of brain arteriovenous malformations and cerebral cavernous malformations
Journal of Human Genetics (2023)
-
BMP10 functions independently from BMP9 for the development of a proper arteriovenous network
Angiogenesis (2023)
-
Mutational spectrum of syndromic genes in sporadic brain arteriovenous malformation
Chinese Neurosurgical Journal (2022)
-
Extracranial arteriovenous malformations demonstrate dysregulated TGF-β/BMP signaling and increased circulating TGF-β1
Scientific Reports (2022)