Abstract
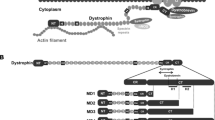
Two lines of transgenic mdx mice have been generated that express a 71 kD non-muscle isoform of dystrophin (Dp71) in skeletal muscle. This isoform contains the cysteine-rich and Oterminal domains of dystrophin, but lacks the N-terminal actin-binding and central spectrin-like repeat domains. Dp71 was associated with the sarcolemma membrane, where it restored normal expression and localization of all members of the dystrophin-associated glycoprotein complex. However, the skeletal muscle pathology of the transgenic mdx mice remained severe. These results indicate that the dystrophin C terminus cannot function independently to prevent dystrophic symptoms and confirms predictions based on patient data that both the N and C-terminal domains are required for normal dystrophin function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hoffman, E.P., Brown, R.H. Jr. & Kunkel, L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987).
Monaco, A.P. et al. Isolation of candidate cDNA clones for portions of the Duchenne muscular dystrophy gene. Nature 323, 646–650 (1986).
Ahn, A.H. & Kunkel, L.M. The structural and functional diversity of dystrophin. Nature Genet. 3, 283–291 (1993).
Byers, T.J., Lidov, H.G.W. & Kunkel, L.M. An alternative dystrophin transcript specific to peripheral nerve. Nature Genet. 4, 77–81 (1993).
Lederfein, D. et al. A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscie tissues. Proc. natn. Acad. Sci. U.S.A. 89, 5346–5350 (1992).
Koenig, M., Monaco, A.P. & Kunkel, L.M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–226 (1988).
Way, M., Pope, B., Cross, R.A., Kendrick-Jones, J. & Weeds, A.G. Expression of the N-terminal domain of dystrophin in E. coil and demonstration of binding to F-actin. FEBS Lett. 301, 243–245 (1992).
Senter, L. et al. Interaction ofdystrophin with cytoskeletal proteins: Binding to talin and actin. Biochem. Biophys. Res. Commun. 192, 899–904 (1993).
Corrado, K., Mills, P.L. & Chamberlain, J.S. Deletion analysis of the dystrophin actin binding domain. FEBS Lett. 344, 255–260 (1994).
Ervasti, J.M. & Campbell, K.P. Membrane organization of the dystrophin glycoprotein complex. Cell 66, 1121–1131 (1991).
Ervasti, J.M. & Campbell, K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. cell Biol. 122, 809–823 (1993).
Suzuki, A., Yoshida, M., Yamamoto, H. & Ozawa, E. Glycoprotein-binding site of dystrophin is confined to the cysteine-rich domain and the first half of the carboxy-terminal domain. FEBS Lett. 308, 154–160 (1992).
Suzuki, A. et al. Molecular organization at the glycoprotein-complex-binding site of dystrophin — Three dystrophin-associated proteins bind directly to the carboxy-terminal portion of dystrophin. Eur. J. Biochem. 220, 283–292 (1994).
England, S.B. et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343, 180–182 (1990).
Passos-Bueno, M.R., Vainzof, M., Marie, S.K. & Zatz, M. Half the dystrophin gene is apparently enough for a mild clinical course: confirmation of its potential use for gene therapy. Hum. molec. Genet. 3, 919–922 (1994).
Koenig, M. et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am. J. hum. Genet. 45, 498–506 (1989).
Prior, T.W. et al. A missense mutation in the dystrophin gene in a Duchenne muscular dystrophy patient. Nature Genet. 4, 357–360 (1993).
Malhotra, S.B. et al. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science 242, 755–759 (1988).
Hoffman, E.P. et al. Is the carboxyl-terminus of dystrophin required for membrane association? A novel, severe case of Duchenne muscular dystrophy. Ann. Neurol. 30, 605–610 (1991).
Matsumura, K. et al. Deficiency of dystrophin-associated proteinsin Duchenne muscular dystrophy patients lacking COOH-terminal domains of dystrophin. J. clin. Invest. 92, 866–871 (1993).
Sicinski, P. et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244, 1578–1580 (1989).
Ohlendieck, K. & Campbell, K.P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J. Cell Biol. 115, 1685–1694 (1991).
Ohlendieck, K., et al. Duchenne muscular dystrophy: Deficiency of dystrophin associated proteins in the sarcolemma. Neurology 43, 795–800 (1993).
Rapaport, D. et al. Characerization and cell type distribution of a novel, major transcript of the Duchenne muscular dystrophy gene. Differentiation 49, 187–193 (1992).
Ibraghimov-Beskrovnaya, O. et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 (1992).
Jaynes, J.B., Chamberlain, J.S., Buskin, J.N., Johnson, J.E. & Hauschka, S.D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Molec. cell. Biol. 6, 2855–2864 (1986).
Johnson, J.E., Wold, B.J. & Hauschka, S.D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Molec. cell. Biol. 9, 3393–3399 (1989).
Cox, G.A., Phelps, S.F., Chapman, V.M. & Chamberlain, J.S. New mdx mutation disrupts expression of muscle and non muscle isoforms of dystrophin. Nature Genet. 4, 87–93 (1993).
Feener, C.A., Koenig, M. & Kunkel, L.M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature 338, 509–511 (1989).
Bies, R.D. et al. Human and murine dystrophin mRNA transcripts are differentially expressed during skeletal muscle, heart, and brain development. Nucl. Acids Res. 20, 1725–1731 (1992).
Bennett, V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol. Rev. 70, 1029–1065 (1990).
Luise, M. et al. Dystrophin is phosphorylated by endogenous protein kinases. Biochem. J. 293, 243–247 (1993).
Milner, R.E., Busaan, J.L., Holmes, C.F.B., Wang, J.H. & Michalak, M. Phosphorylation ofdystrophin. The carboxyl-terminal region of dystrophin is a substrate for in vitro phosphorylation by p34cdc2 protein kinase. J. biol. Chem. 268, 21901–21905 (1993).
Madhavan, R. & Jarrett, H.W. Calmodulin-activated phosphorylation of dystrophin. Biochemistry 33, 5797–5804 (1994).
Tinsley, J.M. et al. Primary structure of dystrophin-related protein. Nature 360, 591–593 (1992).
Tanaka, H., Ishiguro, T., Eguchi, C., Saito, K. & Ozawa, E. Expression of a dystrophin-related protein associated with the skeletal muscle cell membrane. Histochemistry 96, 1–5 (1991).
Ohlendieck, K. et al. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 7, 499–508 (1991).
Milke, T., Miyatake, M., Zhao, J., Yoshioka, K. & Uchino, M. Immunohistochemical dystrophin reaction in synaptic regions. Brain Dev. 11, 344–346 (1989).
Shimizu, T., Matsumura, K., Sunada, Y. & Mannen, T. Dense immunostaining of both neuromuscular and myotendon junctions with an anti-dystrophin antibody. Biomed. Res. 10, 405–409 (1989).
Lyons, P.R. & Slater, C.R. Structure and function of the neuromuscular junction in young adult mdx mice. J. Neurocyt. 20, 969–981 (1991).
Partridge, T. Animal models of muscular dystrophy — What can they teach us? Neuropathol. Appl. Neurobiol. 17, 353–363 (1991).
Wagner, K.R., Cohen, J.B. & Huganir, R.L. The 87K postsynaptic membrane protein from torpedo is a protein-tyrosine kinase substrate homologous to dystrophin. Neuron 10, 511–522 (1993).
Cox, G.A. et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 384, 725–729 (1993).
Rafael, J.A. et al. Prevention of dystrophic pathology in mdx mice by a truncated dystrophin isoform. Hum. molec. Genet. 3, 1725–1733 (1994).
Roberds, S.L et al. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell 78, 625–633> (1994).
Tome, F.M.S. et al. Congenital muscular dystrophy with merosin deficiency. C. R. Acad. Sci. Paris 317, 351–357 (1994).
Passos-Bueno, M.R. et al. Genetic heterogeneity for Duchenne-like muscular dystrophy (DLMD) based on linkage and 50 DAG analysis. Hum. molec. Genet. 2, 1945–1947 (1993).
Greenberg, D.S., Sunada, Y., Campbell, K.P., Yaffe, D. & Nudel, U. Nature Genet. 8, 340–344 (1994).
Ohlendieck, K. & Campbell, K.P. Dystrophin constitutes 5% of membrane cytoskeleton in skeletal muscle. FEBS Lett. 283, 230–234 (1991).
Hogan, B., Constantini, F. & Lacey, E. Manipulating the mouse embryo: a laboratory manual. (Cold Spring Harbor Laboratory Press, New York, (1986).
Chamberlain, J.S., Phelps, S.F., Cox, G.A., Maichele, A.J. & Greenwood, A.D., PCR Analysis of muscular dystrophy in mdx mice. in Molecular and cell biology of muscular dystrophy, (ed. Partridge T.), 167–189 (Chapman & Hall, London, (1993).
Lee, C.C., Pearlman, J.A., Chamberlain, J.S. & Caskey, C.T. Expression of recombinant dystrophin and its localization to the cell membrane. Nature 349, 334–336 (1991).
Ausubel, F.M. Current protocols in molecular biology (John Wiley & Sons, Inc., New York, (1987).
Harlow, E. & Lane, D.P. Antibodies: A laboratory manual Cold Spring Harbor Laboratory Press, New York, (1988).
Bulman, D.E., Murphy, E.G., Zubrzycka-Gaarn, E.E., Worton, R.G. & Ray, P.N. Differentiation of Duchenne and Becker muscular dystrophy phenotypes with amino- and carboxy-terminal antisera specific for dystrophin. Am. J. hum. Genet. 48, 295–304 (1991).
Ohlendieck, K., Ervasti, J.M., Snook, J.B. & Campbell, K.P. Dystrophin glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J. Cell Biol. 112, 135–148 (1991).
Roberds, S.L., Anderson, R.D., Ibraghimov-Beskrovnaya, O. & Campbell, K.P. Primary structure and muscle-specific expression of the 50-kDa dystrophin-associated glycoprotein (Adhalin). J. Biol. Chem. 268, 23739–23742 (1993).
Sambrook, J., Fritsch, E.F., Maniatis, T. Molecular cloning: a laboratory manual, 2nd ed. (Cold Spring Harbor Laboratory Press, New York, (1989).
Meisenhelder, J. & Hunter, T. Phosphorylation of phospholipase C in vivo and in vitro . Methods Enzymol. 197, 288–305 (1991).
Chamberlain, J.S. et al. Mouse dystrophin cDNA Sequence: Genbank accession ♯M68859.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cox, G., Sunada, Y., Campbell, K. et al. Dp71 can restore the dystrophin-associated glycoprotein complex in muscle but fails to prevent dystrophy. Nat Genet 8, 333–339 (1994). https://doi.org/10.1038/ng1294-333
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1294-333
This article is cited by
-
The Shortest Isoform of Dystrophin (Dp40) Interacts with a Group of Presynaptic Proteins to Form a Presumptive Novel Complex in the Mouse Brain
Molecular Neurobiology (2012)
-
Dystrophin Dp71: The Smallest but Multifunctional Product of the Duchenne Muscular Dystrophy Gene
Molecular Neurobiology (2012)
-
Transgenic overexpression of γ-cytoplasmic actin protects against eccentric contraction-induced force loss in mdx mice
Skeletal Muscle (2011)
-
Dystrophin Dp71 is Critical for Stability of the DAPs in the Nucleus of PC12 Cells
Neurochemical Research (2010)
-
Restoration of all dystrophin protein interactions by functional domains in trans does not rescue dystrophy
Gene Therapy (2006)