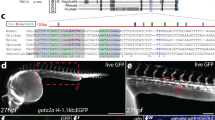
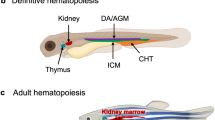
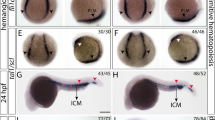
Abstract
Many human anaemias are caused by defects in haemoglobin synthesis. The zebrafish mutant sauternes (sau) has a microcytic, hypochromic anaemia, suggesting that haemoglobin production is perturbed. During embryogenesis, sau mutants have delayed erythroid maturation and abnormal globin gene expression. Using positional cloning techniques, we show that sau encodes the erythroid-specific isoform of δ-aminolevulinate synthase (ALAS2; also known as ALAS-E), the enzyme required for the first step in haem biosynthesis. As mutations in ALAS2 cause congenital sideroblastic anaemia (CSA) in humans, sau represents the first animal model of this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lux, S.E. in Blood: Principles and Practice of Hematology (eds Handin, R.I., Stossel, T.P. & Lux, S.E.) 1383–1398 (J.B. Loppincott, Philadelphia, 1995).
Bridges, K.R. & Seligman, P.A. in Blood: Principles and Practice of Hematology (eds Handin, R.I., Stossel, T.P. & Lux, S.E.) 1433–1472 (J.B. Lippincott, Philadelphia, 1995).
Fleming, M.D. et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genet. 16, 383–386 (1997).
Fleming, M.D. et al. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl Acad. Sci. USA 95, 1148–1153 (1998).
Cotter, P.D., Baumann, M. & Bishop, D.F. Enzymatic defect in "X-linked" sideroblastic anemia: molecular evidence for erythroid δ-aminolvulinate synthase deficiency. Proc. Natl Acad. Sci. USA 89, 4028– 4032 (1992).
Bottomley, S.S., May, B.K., Cox, T.C., Cotter, P.D. & Bishop, D.F. Molecular defects of erythroid 5-aminolevulinate synthase in X-linked sideroblastic anemia. J. Bioenerg. Biomembr. 27, 161–168 (1995).
Sassa, S. & Kappas, A. in Blood: Principles and Practice of Hematology (eds Handin, R.I., Stossel, T.P. & Lux, S.E.) 1388–1398 (J.B. Lippincott, Philadelphia, 1995).
Orkin, S.H. in The Molecular Basis of Blood Diseases (ed. Dyson, J.) 106–122 (W.B. Saunders, Philadelphia, 1987).
Thein, S.L., Wood, W.G., Wickramasinghe, S.N. & Galvin, M.C. ß-thalassemia unlinked to the ß-globin gene in an English family. Blood 82, 961–967 (1993).
Picketts, D.J. et al. ATRX encodes a novel member of the SNF2 family of proteins: mutations point to a common mechanism underlying the ATR-X syndrome. Hum. Mol. Genet. 5, 1899–1907 (1996).
Al-Adhami, M.A. & Kunz, Y.W. Ontogenesis of haematopoietic sites in Brachydanio rerio (Hamilton-Buchanan)(Teleostei). Develop. Growth Differ. 19, 171– 179 (1977).
Detrich, H.W.I. et al. Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl Acad. Sci. USA 92, 10713–10717 (1995).
Chan, F.-Y. et al. Characterization of adult α- and β-globin genes in the zebrafish. Blood 89, 688– 700 (1997).
Catton, W.T. Blood cell formation in certain teleost fishes. Blood 6, 39–60 (1951).
Rowley, A.F., Hunt, T.C., Page, M. & Mainwaring, G. in Vertebrate Blood Cells (eds Rowley, A.F. & Ratcliffe, N.A.) 19– 128 (Cambridge University Press, Cambridge, 1988).
Liao, E.C. et al. SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes Dev. 12, 621–626 (1998).
Thompson, M.A. et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 197, 248–269 (1998).
Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996).
Driever, W. et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37– 46 (1996).
Ransom, D.G. et al. Characterization of zebrafish mutants with defects in embryonic hematopoiesis. Development 123, 311– 319 (1996).
Chen, J.-J. & London, I.M. Regulation of protein synthesis by heme-regulated eIF-2α kinase. Trends Biochem. Sci. 20, 105–109 (1995).
Hunt, T., Vanderhoff, G. & London, I.M. Control of globin synthesis: the role of heme. J. Mol. Biol. 66, 471–481 (1972).
Fukuda, Y., Fujita, H., Garbaczewski, L. & Sassa, S. Regulation of ß-globin mRNA accumulation by heme in DMSO-sensitive and DMSO-resistant murine erythroleukemia cells. Blood 83, 1662–1667 (1994).
Dabney, B.J. & Beaudet, A.L. Increase in globin chains and globin mRNA in erythroleukemia cells in response to hemin. Arch. Biochem. Biophys. 179, 106–112 (1977).
Burger, P.E., Dowdle, E.B., Lukey, P.T. & Wilson, E.L. Basic fibroblast growth factor antagonizes transforming growth factor ß-mediated erythroid differentiation in K562 cells. Blood 83, 1808–1812 (1994).
Streisinger, G., Walker, C., Dower, N., Knauber, D. & Singer, F. Production of clones of homozygous diploid zebrafish (Brachydanio rerio). Nature 291, 293–296 (1981).
Johnson, S.L., Africa, D., Horne, S. & Postlethwait, J.H. Half-tetrad analysis in zebrafish: mapping the ros mutation and the centromere of linkage group I. Genetics 139, 1727– 1735 (1995).
Goff, D.J. et al. Identification of polymorphic simple sequence repeats in the genome of the zebrafish. Genomics 14, 200–202 (1992).
Johnson, S.L. et al. Centromere-linkage analysis and consolidation of the zebrafish genetic map. Genetics 142, 1277– 1288 (1996).
Postlethwait, J.H. et al. Vertebrte genome evolution and the zebrafish gene map. Nature Genet. 18, 345– 349 (1998).
Knapik, E.W. et al. A microsatellite genetic linkage map for zebrafish. Nature Genet. 18, 338–343 (1998).
Postlethwait, J.H. et al. A genetic linmage map for the zebrafish. Science 264, 699–703 (1994).
Riddle, R.D., Yamamoto, M. & Engel, J.D. Expression of δ-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc. Natl Acad. Sci. USA 86, 792– 796 (1989).
Bottomley, S.S., Healy, H.M., Brandenburg, M.A. & May, B.K. 5-aminolevulinate synthase in sideroblastic anemias: mRNA and enzyme activity levels in bone marrow cells. Am. J. Hematol. 41, 76–83 (1992).
Yin, X. & Dailey, H.A. Erythroid 5-aminolevulinate synthase is required for erythroid differentiation in mouse embryonic stem cells. Blood Cells Mol. Dis. 24, 41–53 (1998).
Harigae, H. et al. Deficient heme and globin synthesis inembryonic stem cells lacking the erythroid-specific δ-aminolevulinate synthase gene. Blood 91, 798–805 (1998).
Porter, P.N., Meints, R.H. & Mesner, K. Enhancement of erythroid colony growth in culture by hemin. Exp. Hematol. 7, 11 (1979).
Cox, T.C. et al. X-linked pyridoxine-responsive sideroblastic anemia due to a THR388-to ser substitutionin erythroid 5-aminolevulinate synthase. N. Engl. J. Med. 330, 675–679 (1994).
Cotter, P.D., Rucknagel, D.L. & Bishop, D.F. X-linked sideroblastic anemia: identification of the mutation in the erythroid-specific δ-aminolevulinate synthase gene (ALAS2) in the original family described by Cooley. Blood 84, 3915–1924 (1994).
White, J.M., Brain, M.C. & Ali, M.A.M. Globin synthesis in sideroblastic anaemia. Br. J. Haematol. 20, 263–275 (1971).
Peters, W.E., May, A. & Jacobs, A. Globin chain synthesis ratios in sideroblastic anaemia. Br. J. Haematol. 53, 201–209 (1983).
Gomori, G. Microtechnical demonstration of iron. Am. J. Pathol. 12, 655–663 (1936).
Feder, J.N. et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genet. 13, 399–408 (1996).
Barron, R., Grace, N.D., Sherwood, G. & Powell, L.W. Iron overload complicating sideroblastic anemia—is the gene for hemochromatosis responsible. Gastroenterology 96, 1204– 1206 (1989).
Yaouanq, J., Grosbois, B., Jouanolle, A.M., Goasguen, J. & Leblay, R. Haemochromatosis Cys282Tyr mutation in pyridoxine-responsive sideroblastic anaemia. Lancet 349, 1475–1476 (1997).
Zhang, J., Talbot, W.S. & Schier, A.F. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92, 241–251 (1998).
Wang, H., Long, W., Marty, S.D., Sassa, S. & Lin, S. A zebrafish model of hepatoerythropoietic porphyria. Nature Genet. 20, 239–243 (1998).
Westerfield, M. The Zebrafish Book (Univ. of Oregon Press, Eugene, 1993).
Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B. & Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253– 310 (1995).
Gong, Z. et al. Rapid identification and isolation of zebrafish cDNA clones. Gene 201, 87–98 (1997).
Sassa, S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J. Exp. Med. 143, 305–315 (1976).
Zhong, T.P. et al. Zebrafish genomic library in yeast artificial chromosomes. Genomics 48, 136–138 (1998).
Ota, T. & Amemiya, C.T. A nonradioactive method for improved restriction analysis and fingerprinting of large P1 artificial chromosome clones. Genet. Anal. 12, 173– 178 (1996).
Silverman, G.A. in Methods in Molecular Biology: YAC Protocols (ed. Markie, D.) 145–155 (Humana Press, Totowa, 1998).
Bates, G. in Methods in Molecular Biology: YAC protocols (ed. Markie, D.) 139–144 (Humana Press, Totowa, 1998).
White, J.M. & Ali, M.A.M. Globin synthesis in sideroblastic anaemia. Br. J. Haematol. 24, 481– 489 (1973).
Wheby, M.S. in Fundamentals of Clinical Hematology (ed. Thorup, O.A. Jr) 212– 243 (W.B. Saunders, Philadelphia, 1987).
Acknowledgements
We thank A. Deconinck, S. Ganiatsas and P. Mead for critical review of this manuscript; V. Deane, L. Garbaczewski, C. Rogers and B. Vail for technical assistance. We thank P. Haffter and C. Nusslein-Volhard for providing zebrafish blood mutants before publication, C. Amemiya and G. Silverman for assistance in working with PAC and YAC clones, respectively, and M. Fleming for assistance with prussian blue staining. M. Clark and H. Lerach generously provided kidney cDNA library filters. We also thank Z. Gong for providing the alas1 EST. C. Shackleton provided support for globin structural studies using a VG BioQ mass spectrometer (RR06505). We thank L. Kunkel, S. Orkin and W. Talbot for their support and advice. This work was funded by NIH grants 1RO1 DK53298-01 and P50 DK49216-05 and by USPHS grant DK-32890. H.E.W. is supported through the Northern California Comprehensive Sickle Cell Center Grant No. HL20985. B.H.P. is supported by a Howard Hughes Postdoctoral Fellowship for Physicians. A.C.O. is the recipient of an Anti-Cancer Victoria Postgraduate Research Scholarship. L.I.Z. is an Associate Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brownlie, A., Donovan, A., Pratt, S. et al. Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nat Genet 20, 244–250 (1998). https://doi.org/10.1038/3049
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3049
This article is cited by
-
Zebrafish erythropoiesis and the utility of fish as models of anemia
Stem Cell Research & Therapy (2012)