Abstract
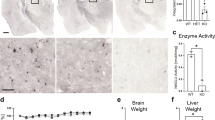
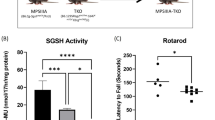
The GM2 gangliosidoses, Tay-Sachs and Sandhoff diseases, are caused by mutations in the HEXA (α-subunit) and HEXB (β-subunit) genes, respectively. Each gene encodes a subunit for the heterodimeric lysosomal enzyme, (β-hexosaminidase A (αβ), as well as for the homodimers β-hexosaminidase B (ββ) and S (αα). In this study, we have produced mice that have both Hexa and Hexb genes disrupted through interbreeding Tay-Sachs1 (Hexa−/−) and Sandhoff2 (Hexb-/-) disease model mice. Lacking both the α and β-subunits these ‘double knockout’ mice displayed a total deficiency of all forms of lysosomal β-hexosaminidase including the small amount of β-hexosaminidase S present in the Sandhoff disease model mice. More surprisingly, these mice showed the phenotypic, pathologic and biochemical features of the mucopolysaccharidoses, lysosomal storage diseases caused by the accumulation of glycosaminoglycans. The mucopolysaccharidosis phenotype is not seen in the Tay-Sachs or Sandhoff disease model mice or in the corresponding human patients3,4,5. This result demonstrates that glycosaminoglycans are crucial substrates for β-hexosaminidase and that their lack of storage in Tay-Sachs and Sandhoff diseases is due to functional redundancy in the β-hexosaminidase enzyme system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Yamanaka, S. et al. Targeted disruption of the Hexa gene results in mice with biochemical and pathologic features of Tay-Sachs disease. Proc. Natl. Acad. Sci. USA 91, 9975–9979 (1994).
Sango, K. et al. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nature Genet. 11, 170–176 (1995).
Applegarth, D.A. & Bozoian, G. Mucopolysaccharide storage in organs of a patient with Sandhoff's disease. Clin. Chim. Acta 39, 269–271 (1972).
Sandhoff, K., Conzelmann, E., Neufeld, E.F., Kaback, M.M. & Suzuki, K. The GM2 gangliosidoses. in The Metabolic Basis of Inherited Disease 1807–1839 (McGraw-Hill, New York, 1989).
Gravel, R.A. et al. The GM2 gangliosidoses. in The Metabolic and Molecular Basis of Inherited Disease (eds Scriver, C.R., Beaudet, A.L, Sly, W.S. & Valle, D.)2839–2879 (McGraw-Hill, New York, 1995).
Taniike, M. et al. Neuropathology of mice with targeted disruption of Hexa gene, a model of Tay-Sachs disease. Acta Neuropathol. 89, 296–304 (1995).
Starr, C.M., Masada, R.I., Hague, C., Skop, E. & Klock, J.C. Fluorophorere-assisted carbohydrate electrophoresis in the separation, analysis, and sequencing of carbohydrates. J. Chromatogr. A 720, 295–321 (1996).
Fratantoni, J.C., Hall, C.W. & Neufeld, E.F. The defect in Hurler's and Hunter's syndromes: Faulty degradation of mucopolysaccharide. Proc. Natl. Acad. Sci. USA 60, 699–706 (1968).
Cantz, M. & Kresse, H. Sandhoff disease: defective glycosaminoglycan catabolism in cultured fibroblasts and its correction by βl-N-acetylhexosaminidase. Eur. J. Biochem. 47, 581–590 (1974).
Neufeld, E.F. & Muenzer, J. The mucopolysaccharidoses. in The Metabolic and Molecular Basis of Inherited Disease (eds Scriver, C. R., Beaudet, A.L., Sly, W.S. & Valle, D.) 2465–2494 (McGraw-Hill, New York, 1995).
Vogler, C. et al. A murine model of mucopotysaccharidosis VII: Gross and microscopic findings in beta-glucuronidase-deficient mice. Am. J. Pathol. 136, 207–217 (1990).
Singh, J., Coppa, G.V. & DiFerrante, N. β-N-Acetylhexosaminidase active on dermatan sulfate. Enzyme 19, 15–23 (1975).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sango, K., McDonald, M., Crawley, J. et al. Mice lacking both subunits of lysosomal β–hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nat Genet 14, 348–352 (1996). https://doi.org/10.1038/ng1196-348
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1196-348