Abstract
Most eukaryotic cell types use a common program to regulate the process of cell division. During mitosis, successful partitioning of the genetic material depends on spatially coordinated chromosome movement and cell cleavage1. Here we characterize a zebrafish mutant, retsina (ret), that exhibits an erythroid-specific defect in cell division with marked dyserythropoiesis similar to human congenital dyserythropoietic anemia. Erythroblasts from ret fish show binuclearity and undergo apoptosis due to a failure in the completion of chromosome segregation and cytokinesis. Through positional cloning, we show that the ret mutation is in a gene (slc4a1) encoding the anion exchanger 1 (also called band 3 and AE1), an erythroid-specific cytoskeletal protein. We further show an association between deficiency in Slc4a1 and mitotic defects in the mouse. Rescue experiments in ret zebrafish embryos expressing transgenic slc4a1 with a variety of mutations show that the requirement for band 3 in normal erythroid mitosis is mediated through its protein 4.1R–binding domains. Our report establishes an evolutionarily conserved role for band 3 in erythroid-specific cell division and illustrates the concept of cell-specific adaptation for mitosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Field, C., Li, R. & Oegema, K. Cytokinesis in eukaryotes: a mechanistic comparison. Curr. Opin. Cell. Biol. 11, 68–80 (1999).
Ransom, D.G. et al. Characterization of zebrafish mutants with defects in embryonic hematopoiesis. Development 123, 311–319 (1996).
Fukuda, M.N. Congenital dyserythropoietic anaemia type II (HEMPAS) and its molecular basis. Baillieres Clin. Haematol. 6, 493–511 (1993).
Wickramasinghe, S.N. Congenital dyserythropoietic anaemias: clinical features, haematological morphology and new biochemical data. Blood Rev. 12, 178–200 (1998).
Liao, E.C. et al. Hereditary spherocytosis in zebrafish riesling illustrates evolution of erythroid β-spectrin structure, and function in red cell morphogenesis and membrane stability. Development 127, 5123–5132 (2000).
Shafizadeh, E. et al. Characterization of zebrafish merlot/chablis as a non-mammalian vertebrate model for severe hereditary elliptocytosis due to protein 4.1 deficiency. Development 129, 4359–4370 (2002).
Alloisio, N. et al. The cisternae decorating the red blood cell membrane in congenital dyserythropoietic anemia (type II) originating from the endoplasmic reticulum. Blood 87, 4433–4439 (1996).
Wei, Y. et al. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 97, 99–109 (1999).
Knapik, E.W. et al. A microsatellite genetic linkage map for zebrafish (Danio rerio) Nat. Genet. 18, 338–343 (1998).
Handin, R.I., Lux, S.E. & Stossel, T.P. Blood: Principles and Practice of Hematology. (J.P. Lippincott, Philadelphia, 1995).
Fritz, A., Rozowski, M., Walker, C. & Westerfield, M. Identification of selected gamma-ray induced deficiencies in zebrafish using multiplex polymerase chain reaction. Genetics 144, 1735–1745 (1996).
Peters, L.L. et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 86, 917–927 (1996).
Peters L.L. et al. Failure of effective reticulocytosis in a new spontaneous band 3 deficient mouse strain (C3H/HeJ-wan) and in a subset of targeted band 3 null mice (B6,129 AE1−/−) suggests the presence of genetic modifiers of band 3 function in red blood cells. Blood 92 Suppl. 1, 301 (1998).
Sekler, I., Lo, R.S. & Kopito, R.R. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J. Biol. Chem. 270, 28751–28758 (1995).
Chernova, M.N. et al. Electrogenic sulfate/chloride exchange in Xenopus oocytes mediated by murine AE1 E699Q. J. Gen. Physiol. 109, 345–360 (1997).
Jons, T. & Drenckhahn, D. Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to erythroid anion exchanger. EMBO J. 11, 2863–2867 (1992).
An, X-L. et al. Modulation of band 3-ankyrin interaction by protein 4.1. J. Biol. Chem. 271, 33187–33191 (1996).
Lombardo, C.R., Willardson, B.M., Low, P.S. Localization of the protein 4.1-binding site on the cytoplasmic domain of erythrocyte membrane band 3. J. Biol. Chem. 267, 9540–9546 (1992).
Shi, Z-T. et al. Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. J. Clin. Invest. 103, 331–340 (1999).
Krauss, S.W. et al. Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division. J. Cell. Biol. 137, 275–289 (1997).
Krauss, S.W. et al. Structural protein 4.1 is located in mammalian centrosomes. Proc. Natl. Acad. Sci. USA 94, 7297–7302 (1997).
Mattagajasingh, S.N. et al. A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein. J. Cell. Biol. 145, 29–43 (1999).
Krauss, S.W. et al. Two distinct domains of protein 4.1 critical for assembly of functional nuclei in vitro. J. Biol. Chem. 277, 44339–44346 (2002).
Perez-Ferreiro, C.M. et al. 4.1R protein associate with interphase microtubules in human T cells. J. Biol. Chem. 276, 44785–44791 (2001).
Gasparini, P. et al. Localization of the congenital dyserythropoietic anemia II locus to chromosome 20q11.2 by genome-wide search. Am. J. Hum. Genet. 61, 1112–1116 (1997).
Iolascon, A. et al. Genetic heterogeneity of congenital dyserythropoietic anemia type II. Blood 92, 2593–2594 (1998).
De Franceschi, L. et al. Decreased band 3 anion transport activity and band 3 clusterization in congenital dyserythropoietic anemia type II. Exp. Hematol. 26, 869–873 (1998).
Brownlie, A. et al. Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nat. Genet. 20, 244–250 (1998).
Farr, C.J., Saiki, R.K., Erlich, H.A., McCormick, F. & Marshall, C.J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc. Natl. Acad. Sci. USA 85, 1629–1633 (1988).
Acknowledgements
We thank K. Dooley, C. Burns, D. Ransom, D. Traver, L. Washburn and C. Birkenmeier for critical review of the manuscript; P. Haffter and C. Nüsslein-Volhard for providing the WIK strain; C. Amemiya, A. Schier and W. Talbot for support and advice; S. Johnson for the DAR and SJD strains; M. Mueckler for SLC2A1 cDNA; M. Listewnik for assistance with the FISH experiments; and N. White and W. Saganic for the zebrafish husbandry. B.H.P. was supported by a Howard Hughes Medical Institutes Post-doctoral Fellowship for Physicians and the US National Institutes of Health. L.I.Z. is an Investigator of the Howard Hughes Medical Institutes. L.L.P. was supported by a US National Cancer Institute Cancer Core Grant to The Jackson Laboratory. The work was supported by funding from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Paw, B., Davidson, A., Zhou, Y. et al. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat Genet 34, 59–64 (2003). https://doi.org/10.1038/ng1137
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1137
This article is cited by
-
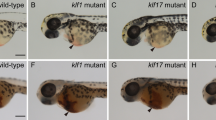
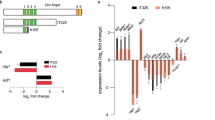
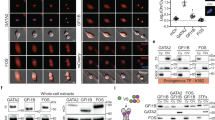
Cooperative contributions of the klf1 and klf17 genes in zebrafish primitive erythropoiesis
Scientific Reports (2023)
-
Whole-exome analysis to detect congenital hemolytic anemia mimicking congenital dyserythropoietic anemia
International Journal of Hematology (2018)
-
Molecular cloning and functional characterization of zebrafish Slc4a3/Ae3 anion exchanger
Pflügers Archiv - European Journal of Physiology (2014)
-
Zebrafish erythropoiesis and the utility of fish as models of anemia
Stem Cell Research & Therapy (2012)
-
The spectrin–ankyrin–4.1–adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life
Protoplasma (2010)