Abstract
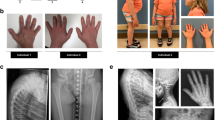
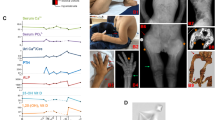
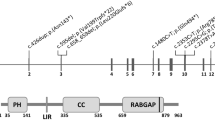
The syndrome of congenital hypoparathyroidism, mental retardation, facial dysmorphism and extreme growth failure (HRD or Sanjad–Sakati syndrome; OMIM 241410) is an autosomal recessive disorder reported almost exclusively in Middle Eastern populations1,2,3. A similar syndrome with the additional features of osteosclerosis and recurrent bacterial infections has been classified as autosomal recessive Kenny–Caffey syndrome4 (AR-KCS; OMIM 244460). Both traits have previously been mapped to chromosome 1q43–44 (refs 5,6) and, despite the observed clinical variability, share an ancestral haplotype, suggesting a common founder mutation7. We describe refinement of the critical region to an interval of roughly 230 kb and identification of deletion and truncation mutations of TBCE in affected individuals. The gene TBCE encodes one of several chaperone proteins required for the proper folding of α-tubulin subunits and the formation of α–β-tubulin heterodimers. Analysis of diseased fibroblasts and lymphoblastoid cells showed lower microtubule density at the microtubule-organizing center (MTOC) and perturbed microtubule polarity in diseased cells. Immunofluorescence and ultrastructural studies showed disturbances in subcellular organelles that require microtubules for membrane trafficking, such as the Golgi and late endosomal compartments. These findings demonstrate that HRD and AR-KCS are chaperone diseases caused by a genetic defect in the tubulin assembly pathway, and establish a potential connection between tubulin physiology and the development of the parathyroid.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Sanjad, S.A., Sakati, N.A., Abu-Osba, Y.K., Kaddoura, R. & Milner, R.D. A new syndrome of congenital hypoparathyroidism, severe growth failure, and dysmorphic features. Arch. Dis. Child. 66, 193–196 (1991).
Richardson, R.J. & Kirk, J.M. Short stature, mental retardation, and hypoparathyroidism: a new syndrome. Arch. Dis. Child. 65, 1113–1117 (1990).
Hershkovitz, E. et al. The new syndrome of congenital hypoparathyroidism associated with dysmorphism, growth retardation, and developmental delay—a report of six patients. Isr. J. Med. Sci. 31, 293–297 (1995).
Khan, T.S.K. et al. Kenny–Caffey syndrome in six Bedouin sibships: autosomal recessive inheritance is confirmed. Am. J. Med. Genet. 69, 126–132 (1997).
Parvari, R. et al. Homozygosity and linkage-disequilibrium mapping of the syndrome of congenital hypoparathyroidism, growth and mental retardation, and dysmorphism to a 1-cM interval on chromosome 1q42-43. Am. J. Hum. Genet. 63, 163–169 (1998).
Diaz, G.A., Khan, K.T. & Gelb, B.D. The autosomal recessive Kenny–Caffey syndrome locus maps to chromosome 1q42–q43. Genomics 54, 13–18 (1998).
Diaz, G.A. et al. Sanjad–Sakati and autosomal recessive Kenny–Caffey syndromes are allelic: evidence for an ancestral founder mutation and locus refinement. Am. J. Med. Genet. 85, 48–52 (1999).
Burge, C. & Karlin, S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 268, 78–94 (1997).
Lewis, S.A., Tian, G., Vainberg, I.E. & Cowan, N.J. Chaperonin-mediated folding of actin and tubulin. J. Cell Biol. 132, 1–4 (1996).
Tian, G. et al. Pathway leading to correctly folded β-tubulin. Cell 86, 287–296 (1996).
Tian, G. et al. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J. Cell Biol. 138, 821–832 (1997).
Pierre, P., Pepperkok, R. & Kreis, T.E. Molecular characterization of two functional domains of CLIP-170 in vivo. J. Cell Sci. 107, 1909–1920 (1994).
Kobe, B. & Deisenhofer, J. The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19, 415–421 (1994).
Radcliffe, P.A., Hirata, D., Vardy, L. & Toda, T. Functional dissection and hierarchy of tubulin-folding cofactor homologues in fission yeast. Mol. Biol. Cell 10, 2987–3001 (1999).
Thyberg, J. & Moskalewski, S. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 246, 263–279 (1999).
Kupfer, A., Dennert, G. & Singer, S.J. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc. Natl Acad. Sci. USA 80, 7224–7228 (1983).
Kupfer, A., Louvard, D. & Singer, S.J. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc. Natl Acad. Sci. USA 79, 2603–2607 (1982).
Meresse, S., Gorvel, J.P. & Chavrier, P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 108, 3349–3358 (1995).
Martin, N. et al. A missense mutation in Tbce causes progressive neuronopathy in mice. Nat. Genet. 32, 443–447 (2002).
Schmalbruch, H., Jensen, H.J., Bjaerg, M., Kamieniecka, Z. & Kurland, L. A new mouse mutant with progressive motor neuronopathy. J. Neuropathol. Exp. Neurol. 50, 192–204 (1991).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Reiner, O. et al. Isolation of a Miller–Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717–721 (1993).
Gleeson, J.G. et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92, 63–72 (1998).
des Portes, V. et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92, 51–61 (1998).
Zhao, C. et al. Charcot–Marie–Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bβ. Cell 105, 587–597 (2001).
Quaderi, N.A. et al. Opitz G/BBB syndrome, a defect of midline development, is due to mutations in a new RING finger gene on Xp22. Nat. Genet. 17, 285–291 (1997).
Schwahn, U. et al. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat. Genet. 19, 327–332 (1998).
Bartolini, F. et al. Functional overlap between retinitis pigmentosa 2 protein and the tubulin-specific chaperone cofactor C. J. Biol. Chem. 14, 14 (2002).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580 (1999).
Rheinwald, J.G. Methods for clonal growth and serial cultivation of normal human epidermal keratinoctyes and mesothelial cells. in Cell Growth and Division: A Practical Approach (ed. Baserga, R.) 81–93 (IRL, Oxford, 1989).
Acknowledgements
This research was supported in part by the Israel Science foundation (to R.P. and R.G.), the US National Institutes of Health (the Mount Sinai Child Health Research Center, Mount Sinai Microscopy Shared Instrument Facility; to B.D.G. and G.A.D.) and the March of Dimes (to G.A.D.). The authors would like to thank the families that participated in the study, S. Zhang and P. Hernandez for excellent technical assistance, T. Volberg for help with the tubulin immunofluoresence staining studies and Y. Ioannou for helpful discussions and critical reading of the manuscript. We gratefully acknowledge the contributions of the Sanger Institute mapping and sequencing groups.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The author declare no competing financial interests.
Rights and permissions
About this article
Cite this article
The HRD/Autosomal Recessive Kenny–Caffey Syndrome Consortium. Mutation of TBCE causes hypoparathyroidism– retardation–dysmorphism and autosomal recessive Kenny–Caffey syndrome. Nat Genet 32, 448–452 (2002). https://doi.org/10.1038/ng1012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1012
This article is cited by
-
Hypoparathyroidism-Retardation-Dysmorphism Syndrome due to a Variant in the Tubulin-Specific Chaperone E Gene as a Cause of Combined Immune Deficiency
Journal of Clinical Immunology (2023)
-
Cortical bone development, maintenance and porosity: genetic alterations in humans and mice influencing chondrocytes, osteoclasts, osteoblasts and osteocytes
Cellular and Molecular Life Sciences (2021)
-
Spatio-temporal distribution of tubulin-binding cofactors and posttranslational modifications of tubulin in the cochlea of mice
Histochemistry and Cell Biology (2020)