Abstract
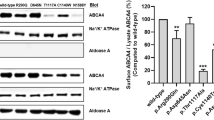
Mutations in the gene encoding ABCR (ABCA4), a photoreceptor-specific ATP-binding cassette (ABC) transporter1,2,3,4, are responsible for autosomal recessive Stargardt disease (STGD), an early onset macular degeneration1,5,6,7,8,9,10,11, and some forms of autosomal recessive cone-rod dystrophy12 and autosomal recessive retinitis pigmentosa12,13,14. Heterozygosity for ABCA4 mutations may also represent a risk factor for age-related macular degeneration15 (AMD), although this idea is controversial7,16,17. An ongoing challenge in the analysis of ABCA4-based retinopathies arises from the observation that most of the ABCA4 sequence variants identified so far are missense mutations that are rare in both patient and control populations. With the current sample size of most sequence variants, one cannot determine statistically whether a particular sequence variant is pathogenic or neutral. A related challenge is to determine the degree to which each pathogenic variant impairs ABCR function, as genotype-phenotype analyses indicate that age of onset and disease severity correlate with different ABCA4 alleles6,8,9,10. To address these questions, we performed a functional analysis of human ABCR and its variants. These experiments reveal a wide spectrum of biochemical defects in these variants and provide insight into the transport mechanism of ABCR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Allikmets, R. et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt's macular dystrophy. Nature Genet. 15, 236–246 (1997).
Azarian, S.M. & Travis, G.H. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt's disease (ABCR). FEBS Lett. 409, 247–252 (1997).
Illing, M., Molday, L.L. & Molday, R.S. The 220 kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272, 10303–10310 (1997).
Sun, H. & Nathans, J. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nature Genet. 17, 15–16 (1997).
Nasonkin, I. et al. Mapping of the rod photoreceptor ABC transporter ABCR to 1p21–p22.1 and identification of novel mutations in Stargardt's disease. Hum. Genet. 102, 21–26 (1998).
Rozet, J.-M. et al. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur. J. Hum. Genet. 6, 291–295 (1998).
Stone, E.M. et al. Allelic variation in ABCR associated with Stargardt disease but not age-related macular degeneration. Nature Genet. 20, 328–329 (1998).
Fishman, G.A. et al. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch. Ophthalmol. 117, 504–510 (1999).
Lewis, R.A. et al. Genotype/phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am. J. Hum. Genet. 64, 422–434 (1999).
Maugeri, A. et al. The 2588G to C mutation in the ABCR gene is a mild frequent founder mutation in the western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am. J. Hum. Genet. 64, 1024–1035 (1999).
Papaioannou, M. et al. An analysis of ABCR mutations in British patients with recessive retinal dystrophies. Inv. Ophthalmol. Vis. Sci. 41, 16–19 (2000).
Cremers F.P.M. et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt disease gene ABCR. Hum. Mol. Genet. 7, 355–362 (1998).
Martinez-Mir, A. et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nature Genet. 18, 11–12 (1998).
Rozet, J.-M. et al. Mutations of the retinal specific ATP binding transporter gene (ABCR) in a single family segregating both autosomal recessive retinitis pigmentosa RP19 and Stargardt disease: evidence of clinical heterogeneity at this locus. J. Med. Genet. 36, 447–451 (1999).
Allikmets, R. et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277, 1805–1807 (1997).
De La Paz, M.A. et al. Analysis of the Stargardt disease gene (ABCR) in age-related macular degeneration. Ophthalmology 106, 1531–1536 (1999).
Souied, E.H. et al. ABCR gene analysis in familial exudative age-related macular degeneration. Inv. Ophthalmol. Vis. Sci. 41, 244–247 (2000).
Ellgaard, L., Molinari, M. & Helenius, A. Setting the standards: quality control in the secretory pathway. Science 286, 1882–1888 (1999).
Sun, H., Molday, R.S. & Nathans, J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ABC transporter responsible for Stargardt disease. J. Biol. Chem. 274, 8269–8281 (1999).
Weng, J. et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 (1999).
Goldberg, A.F.X., Moritz, O.L. & Molday, R.S. Heterologous expression of photoreceptor peripherin/rds and rom-1 in COS-1 cells: assembly, interactions and localization of multisubunit complexes. Biochemistry 34, 14213–14219 (1995).
Loo, T.W. & Clarke, D.M. Covalent modification of human P-glycoprotein mutants containing a single cysteine in either nucleotide-binding fold abolishes drug-stimulated ATPase activity. J. Biol. Chem. 270, 22957–22961 (1995).
Senior, A.E. & Bhagat, S. P-glycoprotein shows strong catalytic cooperativity between the two nucleotide binding sites. Biochemistry 37, 831–836 (1998).
Hung, L.-W. et al. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 396, 703–707 (1998).
Merbs, S.L. & Nathans, J. Photobleaching difference absorption spectra of human cone pigments: quantitative analysis and comparison to other methods. Photochem. Photobiol. 56, 869–881 (1992).
MacKenzie, D., Arendt, A., Hargarve, P., McDowell, J.H. & Molday, R.S. Localization of binding sites for carboxy-terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry 23, 6544–6549 (1984).
Horio, T., Nishikawa, K. & Horiuti, Y. Adenosine triphosphatase: bacterial. Methods Enzymol. 23, 650–654 (1971).
Sung, C.-H., Schneider, B.G., Agarwal, N., Papermaster, D.S. & Nathans, J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc. Natl Acad. Sci. USA 88, 8840–8844 (1991).
Acknowledgements
We thank C. Riley, C. Davenport, M. Kazienko and J. Ptak for DNA synthesis and sequencing; R. Molday for the gift of cells producing Rim3F4; R. Allikmets, J. Lupski and E. Stone for sharing their data on ABCR variants and for helpful discussions; S. Almashanu for advice; and A. Rattner and P. Tong for helpful comments on the manuscript. This work was supported by the Howard Hughes Medical Institute and the National Eye Institute (NIH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, H., Smallwood, P. & Nathans, J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet 26, 242–246 (2000). https://doi.org/10.1038/79994
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/79994
This article is cited by
-
Spectrum of variants associated with inherited retinal dystrophies in Northeast Mexico
BMC Ophthalmology (2024)
-
Mutation screening in genes known to be responsible for Retinitis Pigmentosa in 98 Small Han Chinese Families
Scientific Reports (2017)
-
ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer
Nature Communications (2012)
-
Identification of genetic variation and haplotype structure of the canine ABCA4 gene for retinal disease association studies
Molecular Genetics and Genomics (2010)