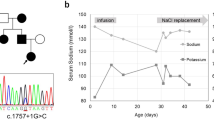
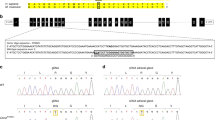
Abstract
Pseudohypoaldosteronism type I (PHA1) is characterized by neonatal renal salt wasting with dehydration, hypotension, hyperkalaemia and metabolic acidosis, despite elevated aldosterone levels. Two forms of PHA1 exist. An autosomal recessive form features severe disease with manifestations persisting into adulthood1. This form is caused by loss-of-function mutations in genes encoding subunits of the amiloride-sensitive epithelial sodium channel (ENaC; refs 2,3 ). Autosomal dominant or sporadic PHA1 is a milder disease that remits with age. Among six dominant and seven sporadic PHA1 kindreds, we have found no ENaC gene mutations, implicating mutations in other genes. As ENaC activity in the kidney is regulated by the steroid hormone aldosterone acting through the mineralocorticoid receptor4, we have screened the mineralocorticoid receptor gene (MLR) for variants and have identified heterozygous mutations in one sporadic and four dominant kindreds. These include two frameshift mutations (one a de novo mutation), two premature termination codons and one splice donor mutation. These mutations segregate with PHA1 and are not found in unaffected subjects. These findings demonstrate that heterozygous MLR mutations cause PHA1, underscore the important role of mineralocorticoid receptor function in regulation of salt and blood pressure homeostasis in humans and motivate further study of this gene for a potential role in blood pressure variation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kuhnle, U. Pseudohypoaldosteronism: mutation found, problem solved? Mol. Cell. Endocrinol. 133, 77–80 (1997).
Chang, S.S. et al. Mutations in subunits of the epithelial sodium channel cause salt-wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nature Genet. 12, 248–253 ( 1996).
Strautnieks, S.S., Thompson, R.J., Gardiner, R.M. & Chung, E. A novel splice-site mutation in the gamma subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type I families. Nature Genet. 13, 248–250 (1996).
Grunder, S. & Rossier, B. A reappraisal of aldosterone effects on the kidney: new insights provided by epithelial sodium channel cloning . Curr. Opin. Nephrol. Hypertens. 6, 35– 39 (1997).
Beato, M. Gene regulation by steroid hormones. Cell 56, 335– 344 (1989).
Shapiro, M.B. & Senapathy, P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 15, 7155–7174 (1987).
Krawczak, M., Reiss, J. & Cooper, D.N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum. Genet. 90, 41–54 (1992).
Berger, S., Cole, T.J., Schmid, W. & Schütz, G. Analysis of glucocorticoid and mineralocorticoid signalling by gene targeting. Endocr. Res. 22, 641–652 (1996).
Zennaro, M.C., Borensztein, P., Jeunemaitre, X., Armanini, D. & Soubrier, F. No alteration in the primary structure of the mineralocorticoid receptor in a family with pseudohypoaldosteronism. J. Clin. Endocrinol. Metab. 79, 32–38 ( 1994).
Komesaroff, P.A., Verity, K. & Fuller, P.J. Pseudohypoaldosteronism: molecular characterization of the mineralocorticoid receptor. J. Clin. Endocrinol. Metab. 79, 27–31 ( 1994).
Arai, K. et al. No apparent mineralocorticoid receptor defect in a series of sporadic cases of pseudohypoaldosteronism. J. Clin. Endocrinol. Metab. 80, 814–817 (1995).
Pascoe, L., Curnow, K.M., Slutsker, L., Rosler, A. & White, P.C. Mutations in the human CYP11B2 (aldosterone synthase) gene causing corticosterone methyloxidase II deficiency. Proc. Natl Acad. Sci. USA 89, 4996–5000 (1992).
Rosler, A. The natural history of salt-wasting disorders of adrenal and renal origin. J. Clin. Endocrinol. Metab. 59, 689–700 (1984).
Koo, W.W. & Gupta, J.M. Breast milk sodium. Arch. Dis. Child. 57, 500–502 (1982).
Sippell W.G., Dorr, H.G., Bidlingmaier F. & Knorr, D. Plasma levels of aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone during infancy and childhood. Ped. Res. 14, 39–46 ( 1980).
Epple, H.J., Schulzke, J.D., Schmitz, H. & Fromm, M. Enzyme and mineralocorticoid receptor-controlled electrogenic Na+ absorption in human rectum in vitro. Am. J. Physiol. 269, G42– G48 (1995).
Gomez-Sanchez, E.P., Zhou, M. & Gomez-Sanchez, C.E. Mineralocorticoids, salt and high blood pressure. Steroids 61, 184–188 ( 1996).
Rodriguez-Soriano, J., Ubetagoyena, M. & Vallo, A. Transtubular potassium concentration gradient: a useful test to estimate renal aldosterone bio-activity in infants and children. Ped. Nephrol. 4, 105–110 (1990).
Bayer, M. & Kutilek, S. A hereditary form of pseudohypoaldosteronism may be manifested in the course of pyelonephritis. Acta Paed. 82, 504 (1993).
Petersen, S. et al. Pseudohypoaldosteronism. case report. Acta Paediatr. Scand. 67, 255–261 (1978).
Zennaro, M.C. et al. Human mineralocorticoid receptor genomic structure and identification of expressed isoforms. J. Biol. Chem. 270, 21016– 21020 (1995).
Shimkets, R.A. et al. Liddle's syndrome: heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell 79, 407–414 (1994).
Hansson, J.H. et al. A de novo missense mutation of the β subunit of the epithelial sodium channel causes hypertension and Liddle's syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc. Natl Acad. Sci. USA 92, 11495–11499 (1995).
Lim-Tio, S.S., Keightley, M.C. & Fuller, P.J. Determinants of specificity of transactivation in the mineralocorticoid or glucocorticoid receptor. Endocrinology 138, 2537–2543 (1997).
Acknowledgements
We thank the families for their contribution to this project, H. Craig and F. Karet for helpful discussions and C. Nelson-Williams for DNA preparation and helpful discussions. Supported by an NIH specialized center of research in hypertension. R.P.L. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geller, D., Rodriguez-Soriano, J., Boado, A. et al. Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism type I. Nat Genet 19, 279–281 (1998). https://doi.org/10.1038/966
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/966
This article is cited by
-
When salt is needed to grow: Answers
Pediatric Nephrology (2021)
-
Recent insights into sodium and potassium handling by the aldosterone-sensitive distal nephron: implications on pathophysiology and drug discovery
Journal of Nephrology (2020)