Abstract
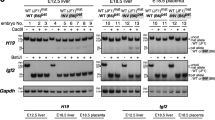
The insulin–like growth factor–II (IGF2) and H19 genes are imprinted in mouse and human, with expression of the paternal IGF2 and maternal H19 alleles. IGF2 undergoes loss of imprinting (LOI) in most Wilms' tumours (WT). We now show that: (i) LOI of IGF2 is associated with a 80–fold down regulation of H19 expression; (ii) these changes are associated with alterations in parental–origin–specific, tissue–independent sites of DNA methylation in the H19 promoter; and (iii) loss of heterozygosity is also associated with loss of H19 expression. Thus, imprinting of a large domain of the maternal chromosome results in a reversal to a paternal epigenotype. These data also suggest an epigenetic mechanism for inactivation of H19 as a tumour suppressor gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Surani, M.A., Reik, W. & Allen, N.D. Transgenes as molecular probes for genomlo imprinting. Trends Genet. 4, 59–62 (1988).
Swain, J.L., Stewart, T.A. & Leder, P. Parental legacy determines methylation and expression of an autosomal transgene: a molecular mechanism for parental imprinting. Cell 50, 719–727 (1987).
Bartolomei, M. The search for imprinted genes. Nature Genet. 6, 4–5 (1994).
DeChiara, T.M., Robertson, E.J. & Efstratiadis, A. Parental imprinting of the mouse insulin-like growth factor-2 gene. Cell 64, 849–859 (1991).
Yee, D. et al. Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res. 48, 6691–6696 (1988).
Lambert, S. et al. TumorIGF-II content in a patient with acolon adenocarcinoma correlates with abnormal expression of the gene. Int. J. Cancer 48, 826–830 (1991).
Shapiro, E.T. et al. Tumor hypoglycemla: relationship to high molecular weight insulin-like growth factor-II. J. clin. Invest. 85, 1672–1679 (1990).
Bartolomei, M., Zemel, S. & Tilghman, S.M. Parental imprinting of the mouse h19 gene. Nature 351, 153–155 (1991).
Glaser, T., Housman, D., Lewis, W.H., Gerhard, D. & Jones, C. A fine-structure deletion map of human chromosome 11p: analysis of J1 series hybrids. Som. Cell molec. Genet 15, 477–501 (1989).
Henry, I. et al. Uniparental paternal disomy In a genetic cancer-predisposing syndrome. Nature 351, 665–667 (1991).
Rainier, S. et al. Relaxation of imprinted genes In human cancer. Nature 362, 747–749 (1993).
Ogawa, O. et al. Relaxation of insulin-like growth factor II gene imprinting Implicated in Wilms' tumour. Nature 362, 749–751 (1993).
Giannoukakis, N., Deal, C., Paquette, J., Goodyer, C.G. & Polychronakos, C. Parental genomic imprinting of the human IGF2 gene. Nature Genet. 4, 98–101 (1993).
Zhang, Y. et al. Imprinting of human H19: allele-specific CpG Methylation, loss of the active allele in Wilrns Tumor, and potential for somatic allele switching. Am. J. hum. Genet 53, 113–124 (1993).
Rachmilewltz, J. et al. Parental imprlnting of the human H19 gene. FEBS Lett. 309, 25–28 (1992).
Hao, Y., Crenshaw, T., Moulton, T., Newcomb, E. & Tycko, B. Tumour-suppressor activity of H19 RNA. Nature 365, 764–767 (1993).
Weksberg, R., Shen, D.R., Fei, Y.L., Song, Q.L. & Squire, J. Disruption of insulin-like growth factor 2 imprinting In Beckwith-Wiedemann syndrome. Nature Genet. 5, 143–150 (1993).
Ogawa, O. et al. Constitutional relaxation of insulin-like growth factor II gene imprinting associated with Wilms' tumour and gigantism. Nature Genet. 5, 408–412 (1993).
Beckwith, J.B., Kiviat, N.B. & Bonadlo, J.F. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Embryol. Devel. 1–36 (1990).
Schroeder, W.T. et al. Nonrandom loss of maternal chromosome 11 alleles in Wilms tumors. Am. J. hum. Genet. 40, 413–420 (1987).
Mannens, M. et al. Molecular nature of genetic changes resulting in loss of heterozygoslty of chromosome 11 in Wilms' tumours. Hum. Genet. 81, 41–48 (1988).
Wilkins, R.J. Genomic imprinting and carcinogenesis. Lancet 1, 329–331 (1988).
Ferguson-Smith, A.C., Sasaki, H., Cattanach, B.M. & Surani, M.A. Parental-origin-specific epigenetic modification of the mouse h19 gene. Nature 362, 751–755 (1993).
Bartolomel, M.S., Webber, A.L., Brunkow, M.E. & Tilghman, S.M. Epigenetic mechanisms underlying the imprinting of the mouse h19 gene. Genes Devel. 7, 1663–1673 (1993).
Feinberg, A.P. & Vogelstein, B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92 (1983).
Goelz, S.E., Vogelstein, B., Hamilton, S.R. & Feinberg, A.P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science 228, 187–190 (1985).
Baylin, S.B. et al. Hypermethylation of the 5′ region of the calcitonin gene is a property of human lymphoid and acute myeloid malignancies. Blood 70, 412–417 (1987).
Henry, I. et al. Somatic mosaicism for partial paternal isodisomy in Wiedemann-Beckwlth syndrome: a post-fertilization event. Bur. J. hum. Genet 1, 19–29 (1993).
Koi, M. et al. Tumor cell growth arrest caused by subchromosomal transferable DNA fragments from human chromosome 11. Science 260, 361–364 (1993).
Feinberg, A. Genomic imprinting and gene activation in cancer. Nature Genet. 4, 110–113 (1993).
Tatof, K.D. & Henikoff, S. Trans-sensing effects from Drosophila to humans. Cell 65, 201–203 (1991).
Cattanach, B.M. & Beechey, C.V. Autosomal and X-chromosome imprinting. Development (Suppl.) 63–72 (1990).
Moore, T. & Haig, D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 7, 45–49 (1991).
Hochberg, A., DeGroot, N., Rachmllewitz, J. & Gonik, B. Genetic imprinting in human evolution: the decisive role of maternal lineage. Med. Hypotheses 41, 355–357 (1993).
Li, E., Beard, C. & Jaenisch, R. Role for DMA methylation in genomic imprinting. Nature 366, 362–365 (1993).
Feinberg, A.P. & Vogelstein, B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132, 6–13 (1983).
Feder, J. et al. A systematic approach for detecting high-frequency restriction fragment length polymorphisms using large genomic probes. Am. J. hum. Genet. 37, 635–649 (1985).
Nelkin, B.D. et al. Structure and expression of a gene encoding human calcitonin and calcitonin gene related peptide. Biochem. Biophys. Res. Commun. 123, 648–655 (1984).
Leppert, M. et al. A partial primary genetic linkage map of chromosome 11. Cytogenet. Cell Genet. 46, 648–1967 (1987).
Wilson, J.T. et al. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucl. Acids Res. 5, 563–581 (1978).
Phillips, J.A. et al. Prenatal diagnosis of sickle cell anemia by restriction endonuclease analysis: Hind III polymorphisms in gamma-globin genes extend test applicability. Proc. natn. Acad. Sci. U.S.A. 77, 2853–2856 (1980).
Tanigami, A. et al. Mapping of 262 DMA markers into 24 intervals on human chromosome 11. Am. J. hum. Genet. 50, 56–64 (1992).
Bell, G.I., Karam, J.H. & Rutter, W.J. Polymorphic DNA region adjacent to the 5′ end of the human insulin gene. Proc. natn. Acad. Sci. U.S.A. 78, 5759–5763 (1981).
Dull, T.J., Gray, A., Hayflick, J.S. & Ullrich, A. Insulin-like growth factor II precursor gene organization In relation to insulin gene family. Nature 310, 777–781 (1984).
Xiang, K., Cox, N.J. & Bell, G.I. Apa I and Sst I RFLP's at the insulin-like growth factor II (IGF2) locus on chromosome 11. Nucl. Acids Res. 16, 3599 (1988).
Brannan, C.I., Dees, E.G., Ingram, R.S. & Tilghman, S.M. The product of the h19 gene may function as an RNA. Molec. cell. Biol. 10, 28–36 (1990).
Redeker, E., Van Moorsel, C.J., Feinberg, A.P. & Mannens, M.A. Taq I and Rsa I polymorphisms in the H19 gene (D11S813E). Hum. molec. Genet. 2, 823 (1993).
Shin, C. & Weinberg, R.A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell 29, 161–169 (1982).
Feinberg, A.P. & Vogelstein, B. Hypomethylation of ras oncogenes in primary human cancers. Biochem. Biophys. Res. Commun. 11, 47–54 (1983).
Saiki, R.K. et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1350–1354 (1985).
Tadokoro, K., Fujii, H., Inoue, T. & Yamada, M. Polymerase chain reaction (PCR) for detection of Apal polymorphism at the Insulin like growth factor II gene (lGF2). Nucl. Acids Res. 19, 6967 (1991).
Rainier, S., Dobry, C.J. & Feinberg, A.P. Dinucleotide repeat polymorphism In the human insulin-like growth factor II (lGF2) gene on chromosome 11. Hum. molec. Genet. 3, 386 (1994).
Zhang, Y. & Tycko, B. Monoallelic expression of the human H19 gene. Nature Genet. 1, 40–44 (1992).
Church, G.M. & Gilbert, W. Genomic sequencing. Proc. natn. Acad. Sci. U.S.A. 81, 1991–1995 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steenman, M., Rainier, S., Dobry, C. et al. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet 7, 433–439 (1994). https://doi.org/10.1038/ng0794-433
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0794-433
This article is cited by
-
Cancer predisposition signaling in Beckwith-Wiedemann Syndrome drives Wilms tumor development
British Journal of Cancer (2024)
-
Blood-derived lncRNAs as biomarkers for cancer diagnosis: the Good, the Bad and the Beauty
npj Precision Oncology (2022)
-
High-risk blastemal Wilms tumor can be modeled by 3D spheroid cultures in vitro
Oncogene (2020)
-
RNA-Seq in 296 phased trios provides a high-resolution map of genomic imprinting
BMC Biology (2019)
-
Lin28 and let-7 regulate the timing of cessation of murine nephrogenesis
Nature Communications (2019)