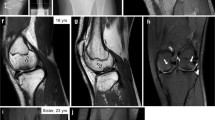
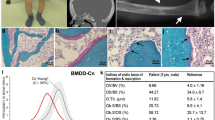
Abstract
The inherited osteolyses or 'vanishing bone' syndromes are a group of rare disorders of unknown etiology characterized by destruction and resorption of affected bones. The multicentric osteolyses are notable for interphalangeal joint erosions that mimic severe juvenile rheumatoid arthritis (OMIMs 166300, 259600, 259610 and 277950). We recently described an autosomal recessive form of multicentric osteolysis with carpal and tarsal resorption, crippling arthritic changes, marked osteoporosis, palmar and plantar subcutaneous nodules and distinctive facies in a number of consanguineous Saudi Arabian families1,2. We localized the disease gene to 16q12–21 by using members of these families for a genome-wide search for homozygous-by-descent microsatellite markers. Haplotype analysis narrowed the critical region to a 1.2-cM region that spans the gene encoding MMP-2 (gelatinase A, collagenase type IV; (ref. 3). We detected no MMP2 enzymatic activity in the serum or fibroblasts of affected family members. We identified two family-specific homoallelic MMP2 mutations: R101H and Y244X. The nonsense mutation effects a deletion of the substrate-binding and catalytic sites and the fibronectin type II-like and hemopexin/TIMP2 binding domains. Based on molecular modeling, the missense mutation disrupts hydrogen bond formation within the highly conserved prodomain adjacent to the catalytic zinc ion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
Al-Mayouf, S.M., Majeed, M., Hugosson, C. & Bahabri, S. New form of idiopathic osteolysis: nodulosis, arthropathy and osteolysis (NAO) syndrome. Am. J. Med. Genet. 93, 5–10 (2000).
Al Aqeel, A. et al. Inherited multicentric osteolysis with arthritis: A variant resembling Torg syndrome in a Saudi family. Am. J. Med. Genet. 93, 11–18 (2000).
Huhtala, P. et al. Completion of the primary structure of the human type IV collagenase preproenzyme and assignment of the gene (CLG4) to the q21 region of chromosome 16. Genomics 6, 554–559 (1990).
Broman, K.W., Murray, J.C., Sheffield, V.C., White, R.L. & Weber, J.L. Comprehensive human genetic maps: Individual and sex-specific variation in recombination. Am. J. Hum. Genet. 63, 861–869 (1998).
Sheffield, V.C. et al. A collection of tri- and tetranucleotide repeat markers used to generate high quality, high resolution human genome-wide linkage maps. Hum. Mol. Genet. 4, 1837–1844 (1995).
Yu, A.E., Murphy, A.N. & Stetler-Stevenson, W.G. in Matrix Metalloproteinases (eds. Parks, W.C. & Mecham, R.P.) 85–113 (Academic Press, San Diego, 1999).
Harris, E.D. Jr. & Krane, S.M. An endopeptidase from rheumatoid synovial tissue culture. Biochim. Biophys. Acta 258, 566–576 (1972).
Creemers, L.B. et al. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol. 17, 35–46 (1998).
Chen, W.T. Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein 49, 59–71 (1996).
Karelina, T.V., Bannikov, G.A. & Eisen, A.Z. Basement membrane zone remodeling during appendageal development in human fetal skin. The absence of type VII collagen is associated with gelatinase-A (MMP-2) activity. J. Invest. Dermatol. 114, 371–375 (2000).
Kanwar, Y.S. et al. Role of membrane-type matrix metalloproteinase 1 (MT-1-MMP), MMP-2, and its inhibitor in nephrogenesis. Am. J. Physiol. 277, F934–F947 (1999).
Morgunova, E. et al. Structure of human pro-matrix metalloproteinase-2: Activation mechanism revealed. Science 284, 1667–1670 (1999).
Murphy, G. et al. The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem. J. 283, 637–641 (1992).
Fridman, R. et al. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J. Biol. Chem. 267, 15398–15405 (1992).
Nguyen, Q. et al. Different domain interactions are involved in the binding of tissue inhibitors of metalloproteinases to stromelysin-1 and gelatinase A. Biochemistry 33, 2089–2095 (1994).
Ye, Q.Z., Johnson, L.L., Yu, A.E. & Hupe, D. Reconstructed 19 kDa catalytic domain of gelatinase A is an active proteinase. Biochemistry 34, 4702–4708 (1995).
Van Wart, H.E. & Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 87, 5578–5582 (1990).
Weber, B.H., Vogt, G., Pruett, R.C., Stohr, H. & Felbor, U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nature Genet. 8, 352–356 (1994).
Ye, S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 7, 623–629 (2000).
Rutter, J.L. et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 58, 5321–5325 (1998).
Brinckerhoff, C.E., Rutter, J.L. & Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 6, 4823–4830 (2000).
Itoh, T. et al. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem. 272, 22389–22392 (1997).
Holmbeck, K. et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99, 81–92 (1999).
Apte, S.S., Fukai, N., Beier, D.R. & Olsen, B.R. The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J. Biol. Chem. 272, 25511–25517 (1997).
Stetler-Stevenson, W.G., Krutzsch, H.C. & Liotta, L.A. Tissue inhibitor of metalloproteinase (TIMP-2): A new member of the metalloproteinase inhibitor family. J. Biol. Chem. 264, 17374–17378 (1989).
Strongin, A.Y. et al. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 270, 5331–5338 (1995).
Butler, G.S. et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J. Biol. Chem. 273, 871–880 (1998).
Zhao, W., Byrne, M.H., Wang, Y. & Krane S.M. Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J. Clin. Invest. 106, 941–949 (2000).
Everts, V. et al. Cathepsin K deficiency, collagen degradation and coupling of bone resorption and formation. J. Bone Miner. Res. 14, S357 (2000).
Kruglyak, L., Daly, M.J., Reeve-Daly, M.P. & Lander, E.S. Parametric and non-parametric linkage analysis: A unified multipoint approach. Am. J. Hum. Genet. 58, 1347–1363 (1996).
Acknowledgements
We thank the families who participated in these studies; M. Palmer, K. Quadrini, P. Prabhudass, R. Kahn, and K. Brown for technical assistance; and I. Visiers for discussions. This work was supported in part by grants from the National Institutes of Health, including a merit award (5 R37 DK34045), research grants (5 R01 DK26824 and R01 NS39893), a grant (5 M01 RR00071) for the Mount Sinai General Clinical Research Center, and a grant (5 P30 HD28822) for the Mount Sinai Child Health Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martignetti, J., Aqeel, A., Sewairi, W. et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet 28, 261–265 (2001). https://doi.org/10.1038/90100
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/90100
This article is cited by
-
Clinical, radiographic and molecular characterization of two unrelated families with multicentric osteolysis, nodulosis, and arthropathy
BMC Musculoskeletal Disorders (2023)
-
Fibroblast growth factor 8 (FGF8) up-regulates gelatinase expression in chondrocytes through nuclear factor-κB p65
Journal of Bone and Mineral Metabolism (2023)
-
Multicentric Carpotarsal Osteolysis: a Contemporary Perspective on the Unique Skeletal Phenotype
Current Osteoporosis Reports (2023)
-
The roles of collagen in chronic kidney disease and vascular calcification
Journal of Molecular Medicine (2021)
-
Coexistence of a novel WISP3 pathogenic variant and an MEFV mutation in an Arabic family with progressive pseudorheumatoid dysplasia mimicking polyarticular juvenile idiopathic arthritis
Pediatric Rheumatology (2020)