Abstract
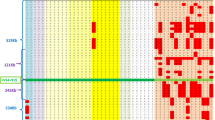
Familial dysautonomia (DYS), the Riley–Day syndrome, is an autosomal recessive disorder characterized by developmental loss of neurons from the sensory and autonomic nervous system. It is limited to the Ashkenazi Jewish population, where the carrier frequency is 1 in 30. We have mapped the DYS gene to chromosome 9q31–q33 by linkage with ten DMA markers in 26 families. The maximum lod score of 21.1 with no recombinants was achieved with D9S58. This marker also showed strong linkage disequilibrium with DYS, with one allele present on 73% of affected chromosomes compared to 5.4% of controls (χ=3142, 15 d.f. p<0.0001). D9S53 and D9S105 represent the closest flanking markers for the disease gene. This localization will permit prenatal diagnosis of DYS in affected families and aid the isolation of the disease gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Riley, C.M., Day, R.L., Greely, D. & Langford, W.S. Central autonomic dysfunction with defective lacrimation. Pediatrics 3, 468–477 (1949).
Axelrod, F.B. & Pearson, J. Congenital sensory neuropathies. Diagnostic distinction from familial dysautonomia. Am. J. Dis. Child. 138, 947–954 (1984).
Axelrod, F.B. Familial dysautonomia and other congenital sensory and autonomic neuropathies. Cellular and Molecular Biology of Neuronal Development (ed. Black I.B.) 331–340 (Plenum Press, New York, 1984).
Brunt, P.W. & McKusick, V.A. Familial dysautonomia, a report of genetic and clinical studies, with a review of the literature. Medicine 49, 343–374 (1970).
Maayan, C., Kaplan, E., Shachar, S., Peleg, O. & Godfrey, S. Incidence of familial dysautonomia in Israel 1977–1981. Clin. Genet. 32, 106–108 (1987).
Blumenfeld, A. et al. Exclusion of familial dysautonomia from more than 60% of the genome. J. med. Genet. 30, 47–52 (1993).
Tanzi, R.E. et al. Genetic linkage map of human chromosome 21. Genomics 3, 129–136 (1988).
Kwiatkowski, D.J. et al. Construction of a GT polymorphism map of human 9q. Genomics 12, 229–240 (1992).
Genome Data Base (GDB) version 4.1, Welch WH Medical Library, Baltimore Maryland 21205.
Gusella, J.F. Location cloning strategy for characterizing genetic defects in Huntington's disease and Alzheimer's disease. FASEB J. 3, 2036–2041 (1989).
Carroll, S.L. et al. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron 9, 779–788 (1992).
Verge, V.M.K. et al. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J. Neurosci. 12, 4011–4022 (1992).
Chao, M.S. Neurotrophin receptors: a window into neuronal differentiation. Neuron 9, 583–593 (1992).
Breakefield, X.O. et al. Structural gene for β-nerve growth factor not defective in familial dysautonomia. Proc. natn. Acad. Sci. U.S.A. 81, 4213–4216 (1984).
Breakefield, X.O. et al. DNA polymorphisms for the nerve growth factor receptor gene exclude its role in familial dysautonomia. Molec. Biol. Med. 3, 483–494 (1986).
Miozzo, M. et al. Human trk proto-oncogene maps to chromosome 1q32–q34. Oncogene 5, 1411–1414 (1990).
Maisonpierre, P.C. et al. Human and rat brain derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics 10, 558–568 (1991).
Berkemeier, L.R., Ozcelik, T., Francke, U. & Rosenthal, A. Human chromosome 19 contains the neurotrophin-5 gene locus and three related genes that may encode novel acidic neurotrophins. Somat. Cell molec. Genet. 18, 233–245 (1992).
Kramer, P.L. et al. Dystonia gene in Ashkenazi Jewish population is located on chromosome 9q32–34. Ann. Neurol. 27, 114–120 (1990).
Ozelius, L.J. et al. Strong allelic association between the torsion dystonia gene (DYT1) and loci on chromosome 9q34 in Ashkenazi Jews. Am. J. hum. Genet. 50, 619–628 (1992).
Walsh, P.S., Metzger, D.A. & Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10, 506–513 (1991).
Ozelius, L.J. et al. A genetic linkage map of human chromosome 9q. Genomics 14, 715–720 (1992).
NIH/CEPH collaborative mapping group. A comprehensive genetic linkage map of the human genome. Science 258, 67–86 (1992).
Williamson, R. et al. Report of the committee and catalogues of cloned and mapped genes, markers formatted for PCR and DNA polymorphisms. Cytogenet. Cell Genet. 58, 1190–1833 (1991).
Wilkie, P.J., Krizman, D.B. & Weber, J.L. Linkage map of human chromosome 9 microsatellite polymorphisms. Genomics 12, 607–609 (1992).
Furlong, R.A. et al. Adinucleotide repeat polymorphism at the D9S109 locus. Nucl. Acids Res. 20, 925 (1992).
Lyall, J.E.W. et al. A dinucleotide repeat polymorphism at the D9S127 locus. Nucl. Acids Res. 20, 925 (1992).
Ozelius, L. et al. Dinucleotide repeat polymorphism for the hexabrachion gene (HXB) on chromosome 9q32–34. Hum. molec. Genet. 1, 141 (1992).
Povey, S. et al. Report on the first international workshop on chromosome 9. Ann. hum. Genet. 56, 167–221 (1992).
Trofatter, J.A., Haines, J.L. & Conneally, P.M. LIPIN: An interactive data entry and management program for LIPED. Am. J. hum. Genet. 39, 147–148 (1986).
Lathrop, G.M., Lalouel, J.M., Julier, C. & Ott, J. Strategies for multi-point linkage analysis in humans. Proc. natn. Acad. Sci. U.S.A. 81, 3443–3446 (1984).
Lander, E.S. et al. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1, 174–181 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blumenfeld, A., Slaugenhaupt, S., Axelrod, F. et al. Localization of the gene for familial dysautonomia on chromosome 9 and definition of DNA markers for genetic diagnosis. Nat Genet 4, 160–164 (1993). https://doi.org/10.1038/ng0693-160
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0693-160
This article is cited by
-
Genetic variability of pain perception and treatment—clinical pharmacological implications
European Journal of Clinical Pharmacology (2011)
-
The Molecular Basis of Familial Dysautonomia: Overview, New Discoveries and Implications for Directed Therapies
NeuroMolecular Medicine (2008)
-
Hereditary sensory and autonomic neuropathies: types II, III, and IV
Orphanet Journal of Rare Diseases (2007)
-
Therapeutic potential and mechanism of kinetin as a treatment for the human splicing disease familial dysautonomia
Journal of Molecular Medicine (2007)
-
Cardiac pacing in patients with familial dysautonomia
Clinical Autonomic Research (2005)