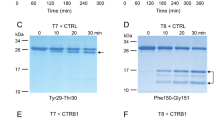
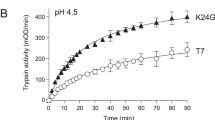
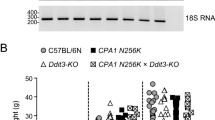
Abstract
Chronic pancreatitis (CP) is a continuing or relapsing inflammatory disease of the pancreas. In approximately one-third of all cases, no aetiological factor can be found, and these patients are classified as having idiopathic disease. Pathophysiologically, autodigestion and inflammation may be caused by either increased proteolytic activity or decreased protease inhibition. Several studies have demonstrated mutations in the cationic trypsinogen gene (PRSS1) in patients with hereditary1,2,3 or idiopathic4 CP. It is thought that these mutations result in increased trypsin activity within the pancreatic parenchyma. Most patients with idiopathic or hereditary CP, however, do not have mutations in PRSS1 (ref. 4). Here we analysed 96 unrelated children and adolescents with CP for mutations in the gene encoding the serine protease inhibitor, Kazal type 1 (SPINK1), a pancreatic trypsin inhibitor. We found mutations in 23% of the patients. In 18 patients, 6 of whom were homozygous, we detected a missense mutation of codon 34 (N34S). We also found four other sequence variants. Our results indicate that mutations in SPINK1 are associated with chronic pancreatitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whitcomb, D.C. et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature Genet. 14, 141– 145 (1996).
Gorry, M.C. et al. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology 113, 1063–1068 ( 1997).
Ferec, C. et al. Mutations in the cationic trypsinogen gene and evidence for genetic heterogeneity in hereditary pancreatitis. J. Med. Genet. 36, 228–232 (1999).
Witt, H., Luck, W. & Becker, M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology 117, 7–10 (1999).
Horii, A. et al. Primary structure of human pancreatic secretory trypsin inhibitor (PSTI) gene. Biochem. Biophys. Res. Commun. 149, 635–641 (1987).
Bartelt, D.C., Shapanka, R. & Greene, L.J. The primary structure of the human pancreatic secretory trypsin inhibitor. Amino acid sequence of the reduced S-aminoethylated protein . Arch. Biochem. Biophys. 179, 189– 199 (1977).
Marchbank, T., Freeman, T.C. & Playford, R.J. Human pancreatic secretory trypsin inhibitor. Digestion 59, 167–174 ( 1998).
Rinderknecht, H. Pancreatic secretory enzymes. in The Pancreas: Biology, Pathobiology, and Disease (eds Go, V.L.W. et al.) 219– 251 (Raven, New York, 1993).
Spielman, R.S., McGinnis, R.E. & Ewens, W.J. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am. J. Hum. Genet. 52, 506–516 (1993).
Falk, C.T. & Rubinstein, P. Haplotype relative risks: an easy reliable way to construct a proper control sample for risk calculations . Ann. Hum. Genet. 51, 227– 233 (1987).
Terwilliger, J.D. A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. Am. J. Hum. Genet. 56, 777–787 ( 1995).
Laskowski, M. Jr & Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 49, 593–626 (1980).
Rinderknecht, H. Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Dig. Dis. Sci. 31, 314–321 (1986).
Sharer, N. et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N. Engl. J. Med. 339, 645– 652 (1998).
Cohn, J.A. et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N. Engl. J. Med. 339, 653–658 (1998).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380, 152– 154 (1996).
Acknowledgements
We thank the families for participation; the members of the Gesellschaft für Pädiatrische Gastroenterologie und Ernährung for providing clinical data and blood samples; C. Güldner and I. Liebner for technical assistance; and F. Rüschendorf for support in computer science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witt, H., Luck, W., Hennies, H. et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 25, 213–216 (2000). https://doi.org/10.1038/76088
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76088
This article is cited by
-
Hereditary Pancreatitis Related to SPINK-1 Mutation. Is There an Increased Risk of Developing Pancreatic Cancer?
Journal of Gastrointestinal Cancer (2023)
-
Expanding ACMG variant classification guidelines into a general framework
Human Genomics (2022)
-
Identification of novel differentially expressed genes in type 1 diabetes mellitus complications using transcriptomic profiling of UAE patients: a multicenter study
Scientific Reports (2022)
-
Evidence-based clinical practice guidelines for chronic pancreatitis 2021
Journal of Gastroenterology (2022)
-
Genetic Testing in Acute and Chronic Pancreatitis
Current Treatment Options in Gastroenterology (2022)