Abstract
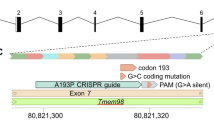
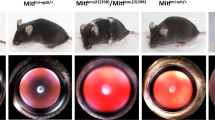
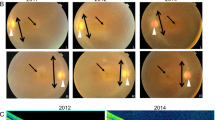
Glaucomas are a major cause of blindness1. Visual loss typically involves retinal ganglion cell death and optic nerve atrophy subsequent to a pathologic elevation of intraocular pressure (IOP). Some human glaucomas are associated with anterior segment abnormalities such as pigment dispersion syndrome (PDS) and iris atrophy with associated synechiae2. The primary causes of these abnormalities are unknown, and their aetiology is poorly understood. We recently characterized a mouse strain (DBA/2J) that develops glaucoma subsequent to anterior segment changes including pigment dispersion and iris atrophy3. Using crosses between mouse strains DBA/2J (D2) and C57BL/6J (B6), we now show there are two chromosomal regions that contribute to the anterior segment changes and glaucoma. Progeny homozygous for the D2 allele of one locus on chromosome 6 (called ipd) develop an iris pigment dispersion phenotype similar to human PDS. ipd resides on a region of mouse chromosome 6 with conserved synteny to a region of human chromosome 7q that is associated with human PDS (ref. 4 ). Progeny homozygous for the D2 allele of a different locus on chromosome 4 (called isa) develop an iris stromal atrophy phenotype (ISA). The Tyrp1 gene is a candidate for isa and likely causes ISA via a mechanism involving pigment production. Progeny homozygous for the D2 alleles of both ipd and isa develop an earlier onset and more severe disease involving pigment dispersion and iris stromal atrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Quigley, H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 80, 389–393 (1996).
Spencer, W.H. Glaucoma. in Ophthalmic Pathology, An Atlas and Textbook (ed. Spencer, W.H.) 438–512 (W.B. Saunders Company, Philadelphia, 1996).
John, S.W.M. et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 39, 951–962 (1998).
Andersen, J.S. et al. A gene responsible for the pigment dispersion syndrome maps to chromosome 7q35-q36. Arch. Ophthalmol. 115, 384–388 (1997).
Ritch, R., Steinberger, D. & Liebmann, J.M. Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am. J. Ophthalmol. 115 , 707–710 (1993).
Richter, C.U., Richardson, T.M. & Grant, W.M. Pigmentary dispersion syndrome and pigmentary glaucoma. A prospective study of the natural history. Arch. Ophthalmol. 104, 211–215 (1986).
Migliazzo, C.V., Shaffer, R.N., Nykin, R. & Magee, S. Long-term analysis of pigmentary dispersion syndrome and pigmentary glaucoma. Ophthalmology 93, 1528–1536 ( 1986).
Farrar, S.M., Shields, M.B., Miller, K.N. & Stoup, C.M. Risk factors for the development and severity of glaucoma in the pigment dispersion syndrome. Am. J. Ophthalmol. 108, 223– 229 (1989).
Shields, M.B. Iridocorneal endothelial Syndromes. In Chandler and Grant's Glaucoma (eds Epstein, D.L., Allingham, R.R. & Schuman, J.S.) 319– 371 (Williams & Wilkins, Baltimore, 1997).
Shields, M.B. & Bourgeois, J.E. Glaucoma associated with primary disorders of the corneal endothelium. In The Glaucomas (eds Ritch, R., Shields, M.B. & Krupin, T.) 957–974 (Mosby Year Book, St. Louis, 1996).
Shields, M.B. Progressive essential iris atrophy, Chandler's syndrome, and the iris nevus (Cogan-Reese) syndrome: a spectrum of disease. Surv. Ophthalmol. 24, 3–20 (1979 ).
Spencer, W., Folberg, R., Eagle, R.C. & Rao, N. Ophthalmic Pathology, An Atlas and Textbook, (W.B. Saunders Company, Philadelphia, 1996).
Shields, M.B. Axenfeld-Rieger syndrome: a theory of mechanism and distinctions from the iridocorneal endothelial syndrome. Trans. Am. Ophthalmol. Soc. 81, 736–784 ( 1983).
Blum, J.V., Allen, J.H. & Holland, M.G. Familial bilateral essential iris atrophy. Trans. Am. Acad. Ophthalmol. Otolaryngol. 66, 493– 500 (1962).
Jampol, L.M., Rosser, M.J. & Sears, M.L. Unusual aspects of progressive essential iris atrophy. Am. J. Ophthalmol. 77, 353– 357 (1974).
Shields, M.B., McCracken, J.S., Klintworth, G.K. & Campbell, D.G. Corneal edema in essential iris atrophy. Ophthalmology 86, 1533–1550 (1979).
Frank-Kamenetzki, S.G. Eine Eigenartige Hereditare Glaukomform it Mangel Des Irisstromas und Geschlechtsgebundener Vererbung. Klin. Monatsbl. Augenheilkd. 74, 133 (1925).
Semina, E.V. et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nature Genet. 14, 392–399 (1996).
Mears, A.J. et al. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am. J. Hum. Genet. 63, 1316–1328 (1998).
Nishimura, D.Y. et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nature Genet. 19, 140–147 (1998).
Heon, E. et al. Linkage of posterior polymorphous corneal dystrophy to 20q11. Hum. Mol. Genet. 4, 485– 488 (1995).
Jackson, I.J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc. Natl Acad. Sci. USA 85, 4392– 4396 (1988).
Bennett, D.C., Huszar, D., Laipis, P.J., Jaenisch, R. & Jackson, I.J. Phenotypic rescue of mutant brown melanocytes by a retrovirus carrying a wild-type tyrosinase-related protein gene. Development 110, 471–475 (1990).
Jackson, I.J. et al. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 11, 527–535 (1992).
Zdarsky, E., Favor, J. & Jackson, I.J. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics 126, 443–449 (1990).
Shibahara, S., Tomita, Y., Yoshizawa, M., Shibata, K. & Tagami, H. Identification of mutations in the pigment cell-specific gene located at the brown locus in mouse. Pigment Cell Res. 2, 90–95 (1992).
Johnson, R. & Jackson, I.J. Light is a dominant mouse mutation resulting in premature cell death. Nature Genet. 1, 226–229 (1992).
Manga, P. et al. Rufous oculocutaneous albinism in southern African Blacks is caused by mutations in the TYRP1 gene. Am. J. Hum. Genet. 61, 1095–1101 ( 1997).
Acknowledgements
We thank F. Farley, J. Smith, W. Whitebone, J. Worcester and K. Burns for assistance preparing the manuscript; J. Martin, C. Fickett and C. Callaghan for animal care; D. Harrison for BALB/cByJ mice; B. Taylor for BXD mice and advice; J. Wiggs for helpful discussion; J. Naggert, J. Schimenti and B. Knowles for critical reading of the manuscript; and R. Edwards for assistance configuring the slit-lamp system. This work was supported in part by National Eye Institute grant EY07758, The Foundation Fighting Blindness and grant CA34196 from the National Cancer Institute. SWMJ is an Assistant Investigator of The Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, B., Smith, R., Hawes, N. et al. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice . Nat Genet 21, 405–409 (1999). https://doi.org/10.1038/7741
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7741
This article is cited by
-
A missense mutation in Pitx2 leads to early-onset glaucoma via NRF2-YAP1 axis
Cell Death & Disease (2021)
-
Quantitative trait locus mapping identifies the Gpnmb gene as a modifier of mouse macrophage lysosome function
Scientific Reports (2021)
-
Exome-based investigation of the genetic basis of human pigmentary glaucoma
BMC Genomics (2021)
-
Of Mice and Monkeys: Neuroprotective Efficacy of the p38 Inhibitor BIRB 796 Depends on Model Duration in Experimental Glaucoma
Scientific Reports (2020)
-
Protect, Repair, and Regenerate: Towards Restoring Vision in Glaucoma
Current Ophthalmology Reports (2020)