Abstract
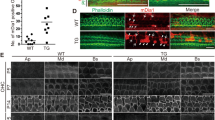
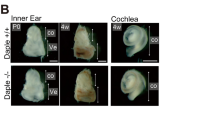
The mammalian inner ear contains organs for the detection of sound and acceleration, the cochlea and the vestibule, respectively. Mechanosensory hair cells within the neuroepithelia of these organs transduce mechanical force generated by sound waves or head movements into neuronal signals. Defects in hair cells lead to deafness and balance defects1,2,3. Hair cells have stereocilia that are indispensable for mechanosensation, but the molecular mechanisms regulating stereocilia formation are poorly understood4,5,6. We show here that integrin α8β1, its ligand fibronectin and the integrin-regulated focal adhesion kinase (FAK) co-localize to the apical hair-cell surface where stereocilia are forming. In mice homozygous for a targeted mutation of Itga8 (encoding the α8 subunit; ref. 7), this co-localization is perturbed and hair cells in the utricle, a vestibular subcompartment, lack stereocilia or contain malformed stereocilia. Most integrin-α8β1–deficient mice die soon after birth due to kidney defects. Many of the survivors have difficulty balancing, consistent with the structural defects of the inner ear. Our data suggest that integrin α8β1, and potentially other integrins, regulates hair-cell differentiation and stereocilia maturation. Mutations affecting matrix molecules cause inherited forms of inner ear disease1,2,3,8 and integrins may mediate some effects of matrix molecules in the ear; thus, mutations in integrin genes may lead to inner-ear diseases as well.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Petit, C. Genes responsible for human hereditary deafness: symphony of a thousand. Nature Genet. 14, 385–391 (1996).
Corwin, J.T. Identifying the genes of hearing, deafness, and disequilibrium. Proc. Natl Acad. Sci. USA 95, 12080–12082 (1998).
Cremers, F.P.M. Genetic causes of hearing loss. Curr. Opin. Neurol. 11, 11–16 (1999).
Lim, D.J. & Anniko, M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol. Suppl. (Stockh) 422, 1–69 (1985).
Corwin, J.T. & Warchol, M.E. Auditory hair cells: structure, function, development, and regeneration. Annu. Rev. Neurosci. 14, 301–333 (1991).
Tilney, L.G., Tilney, M.S. & DeRosier, D.J. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 8, 257–274 (1992).
Müller, U. et al. Integrin α8β1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell 88, 603–613 (1997).
McGuirt, W.T. et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nature Genet. 23, 413–419 (1999).
Hynes, R.O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 (1992).
Sonnenberg, A. Integrins and their ligands. Curr. Top. Microbiol. Immunol. 184, 7–35 (1993).
Legan, P.K. & Richardson, G.P. Extracellular matrix and cell adhesion molecules in the developing inner ear. Semin. Cell Dev. Biol. 8, 217–224 (1997).
Schlaepfer, D.D. & Hunter, T. Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell. Biol. 8, 151–157 (1998).
Schoenwaelder, S.M. & Burridge, K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 11, 274–286 (1999).
Lynch, E.D. et al. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science 278, 1315–1318 (1997).
Ruben, R.J. Development of the inner ear of the mouse: a radioautographic study of terminal mitosis. Acta Otolaryngol. Suppl. (Stock) 220, 1–44 (1967).
Zheng, J.L. & Gao, W.Q. Analysis of rat vestibular hair cell development and regeneration using calretinin as an early marker. J. Neurosci. 17, 8270–8282 (1997).
Hasson, T. et al. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 137, 1287–1307 (1997).
Müller, U., Bossy, B., Venstrom, K. & Reichardt, L.F. Integrin α8β1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol. Biol. Cell 6, 433–448 (1995).
Schnapp, L.M. et al. The human integrin α8β1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 270, 23196–23202 (1995).
Denda, S., Reichardt, L.F. & Müller, U. Identification of osteopontin as a novel ligand for the integrin α8β1 and potential roles of this integrin-ligand interaction in kidney morphogenesis. Mol. Biol. Cell 9, 1425–1435 (1998).
Varnum-Finney, B. et al. The integrin receptor α8β1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron 14, 1213–1222 (1995).
Whitlon, D.S., Zhang, X. & Kusakabe, M. Tenascin-C in the cochlea of the developing mouse. J. Comp. Neurol. 406, 361–374 (1999).
Lopez, C.A. et al. Osteopontin expression detected in adult cochleae and inner ear fluids. Hearing Res. 85, 210–222 (1995).
Takemura, T. et al. Localization of osteopontin in the otoconial organs of adult rats. Hearing Res. 79, 99–104 (1994).
Cosgrove, D., Kornak, J.M. & Samuelson, G. Expression of basement membrane type IV collagen chains during postnatal development in the murine cochlea. Hearing Res. 100, 21–32 (1996).
Schaeren-Wiemers, N. & Gerfin-Moser, A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431–440 (1993).
Acknowledgements
We thank J.M. Bergelson, T. Hasson, R.O. Hynes, J.H. Miner and L.F. Reichardt for reagents; P. Caroni, A. Kralli, D. Monard and P. Mathias for critical reading of the manuscript; members of the laboratory for comments; and T. Laux, P. Piosik and O. Baldomero for technical advice. This work was funded by the Novartis Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Littlewood Evans, A., Müller, U. Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin α8β1. Nat Genet 24, 424–428 (2000). https://doi.org/10.1038/74286
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/74286
This article is cited by
-
Acute Noise Causes Down-Regulation of ECM Protein Expression in Guinea Pig Cochlea
Molecular Biotechnology (2023)
-
Fibroblast-Secreted Phosphoprotein 1 Mediates Extracellular Matrix Deposition and Inhibits Smooth Muscle Cell Contractility in Marfan Syndrome Aortic Aneurysm
Journal of Cardiovascular Translational Research (2022)
-
Essential Role of Sptan1 in Cochlear Hair Cell Morphology and Function Via Focal Adhesion Signaling
Molecular Neurobiology (2022)
-
A combined genome-wide association and molecular study of age-related hearing loss in H. sapiens
BMC Medicine (2021)
-
Establishment of planar cell polarity is coupled to regional cell cycle exit and cell differentiation in the mouse utricle
Scientific Reports (2017)