Abstract
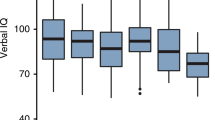
Neurofibromatosis type I (NF1) is one of the most commonly inherited neurological disorders in humans, affecting approximately one in 4,000 individuals1–3. NF1 results in a complex cluster of developmental and tumour syndromes that include benign neurofibromas, hyperpigmentation of melanocytes and hamartomas of the iris. Some NF1 patients may also show neurologic lesions, such as optic pathway gliomas, dural ectasia and aqueduct stenosis1–3. Importantly, learning disabilities occur in 30% to 45% of patients with NF1, even in the absence of any apparent neural pathology. The learning disabilities may include a depression in mean IQ scores, visuoperceptual problems and impairments in spatial cognitive abilities4–9. Spatial learning has been assessed with a variety of cognitive tasks and the most consistent spatial learning deficits have been observed with the Judgement of Line Orientation test4,7,10,11. It is important to note that some of these deficits could be secondary to developmental abnormalities1 and other neurological problems, such as poor motor coordination and attentional deficits9. Previous studies have suggested a role for neurofibromin in brain function. First, the expression of the Nf1 gene is largely restricted to neuronal tissues in the adult12,14. Second, this GTPase activating protein may act as a negative regulator of neurotrophin-mediated signalling15. Third, immunohistochemical studies suggest that activation of astrocytes may be common in the brain of NF1 patients13. Here, we show that the Nf1+/− mutation also affects learning and memory in mice. As in humans, the learning and memory deficits of the Nf1+/− mice are restricted to specific types of learning, they are not fully penetrant, they can be compensated for with extended training, and they do not involve deficits in simple associative learning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gutmann, D.H. & Collins, F.S. Recent progress towards understanding the molecular biology of von Recklinghausen neurofibromatosis. Annl. Neurol. 31, 555–561 (1992).
Huson, S.M. & Hughes, R.A.C. The Neurofibromatoses: A Pathogenic and Clinical Overview, 204–252 (Chapman & Hall, London, 1994).
Riccardi, V.M. Neurofibromatosis: Phenotype, Natural History and Pathogenesis, 195–213 (The Johns Hopkins Press Ltd., Baltimore, 1992).
Eliason, M.J. Neurofibromatosis: implications for learning and behavior. Dev. Behav. Pediatr. 7, 175–179 (1986).
Eliason, M.J. Neuropsychological patterns: neurofibromatosis compared to developmental learning disorders. Neurofibromatosis 1, 17–25 (1988).
Varnhagen, C. et al. Neurofibromatosis and psychological processes. Dev. Behav. Pediatr. 9, 257–265 (1988).
Eldridge, R. et al. Neurofibromatosis type 1 (Recklinghausen's Disease): Neurologic and cognitive assessment with sibling controls. Am. J. Dis. Child 143, 833–837 (1989).
North, K. Neurofibromatosis type 1: review of the first 200 patients in an Australian clinic. J. Child Neurol. 8, 395–402 (1993).
North, K. Joy, P. Yuille, D. Cocks, N. & Hutchins, P. Cognitive function and academic performance in children with neurofibromatosis type 1. Dev. Med. Child. Neurol. 37, 427–436 (1995).
Hofman, K.J. Harris, E.L. Bryan, R.N. & Denckla, M.B. Neurofibromatosis type 1: the cognitive phenotype. J. Pediatr. 124, 1–8 (1994).
Joy, P. Roberts, C. North, K. & de Silva, M. Neuropsychological function and MRI abnormalities in neurofibromatosis type 1. Dev. Med. Child. Neurol. 37, 906–914 (1995).
Datson, M.M. et al. The protein product of the neurofibromatsis type 1 gene is expressed at highest abundance in neurons, schwann cells, and oligodendrocytes. Neuron 8, 415–428 (1992).
Nordlund, M.L. Rizvi, T.A. Brannan, C.I. & Ratner, N. Neurofibromin expression and astrogliosis in neurofibromatosis (type 1) brains. J. Neuropathol. Exp. Neurol. 54, 588–600 (1995).
Nordlund, M. Gu, X. Shipley, M.T. & Ratner, N. Neurofibromin is enriched in the endoplasmic reticulum of CNS neurons. J. Neurosci. 13, 1588–1600 (1993).
Vogel, K.S. Brannan, C.I. Jenkins, N.A. Copeland, N.G. & Parada, L.F. Loss of neurofibromin results in neurotrophin-independent survival of embryonic sensory and sympathetic neurons. Cell 82, 733–742 (1995).
Jacks, T. et al. Tumour predisposition in mice heterozygous for a targeted mutation of NF1. Nature Genet. 7, 353–361 (1994).
Morris, R.G.M. Garrud, P. Rawlins, J.N.P. & O'Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
Sutherland, R.J. Kolb, B. & Whishaw, I.Q. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci. Lett. 31, 271–276 (1982).
Wu, Z.L. et al. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc. Natl. Acad. Sci. USA 92, 220–224 (1995).
Brandeis, R. Brandys, Y. & Yehuda, S. The use of the Morris Water Maze in the study of memory and learning. Intern. J. Neurosci. 48, 29–69 (1989).
Crawley, J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 9, 37–44 (1985).
Easton, D. Ponder, M. Huson, S. & Ponder, B. An analysis of variation in expression of neurofibromatosis (NF) type 1 (NF1): evidence for modifying genes. Am. J. Hum. Genet. 53, 305–313 (1993).
Li, Y. Erzurumlu, R.S. Chen, C. Jhaveri, S. & Tonegawa, S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of Nmdarf knockout mice. Cell 76, 427–437 (1994).
Morris, R.G.M. Ahderson, E. Lynch, G.S. & Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-asparate receptor antagonist, APS. Nature 319, 774–776(1986).
Bliss, T.V.P. & Collingridge, G.L. A synaptic model of memory: Long-term-potentiation. Nature 361, 31–39 (1993).
Kirn, J.J. & Fanselow, M.S. Modality-specific retrograde amnesia of fear. Science 256, 675–677 (1992).
Phillips, R.G. & LeDoux, J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285 (1992).
Bourtchuladze, R. et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element binding protein. Cell 79, 59–68 (1994).
LeDoux, J.E. Clues from the brain. Annu. Rev. Psychol. 46, 209–235 (1995).
Zhong, Y. Mediation of PACAP-like neuropeptide transmission by coactivation of Ras/Raf and cAMP signal transduction pathways in Drosophila. Nature 375, 588–592 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Silva, A., Frankland, P., Marowitz, Z. et al. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet 15, 281–284 (1997). https://doi.org/10.1038/ng0397-281
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0397-281
This article is cited by
-
Improvement of synaptic plasticity and cognitive function in RASopathies—a monocentre, randomized, double-blind, parallel-group, placebo-controlled, cross-over clinical trial (SynCoRAS)
Trials (2023)
-
Using antisense oligonucleotides for the physiological modulation of the alternative splicing of NF1 exon 23a during PC12 neuronal differentiation
Scientific Reports (2021)
-
The impact of RASopathy-associated mutations on CNS development in mice and humans
Molecular Brain (2019)
-
Social support rescues acute stress-induced cognitive impairments by modulating ERK1/2 phosphorylation in adolescent mice
Scientific Reports (2018)
-
Genetic enhancement of Ras-ERK pathway does not aggravate L-DOPA-induced dyskinesia in mice but prevents the decrease induced by lovastatin
Scientific Reports (2018)