Abstract
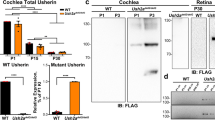
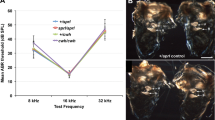
Genes specifically expressed in the inner ear are candidates to underlie hereditary nonsyndromic deafness1. The gene Otog has been isolated from a mouse subtractive cDNA cochlear library2. It encodes otogelin, an N-glycosylated protein that is present in the acellular membranes covering the six sensory epithelial patches of the inner ear: in the cochlea (the auditory sensory organ), the tectorial membrane (TM) over the organ of Corti; and in the vestibule (the balance sensory organ), the otoconial membranes over the utricular and saccular maculae as well as the cupulae over the cristae ampullares of the three semi-circular canals. These membranes are involved in the mechanotransduction process. Their movement, which is induced by sound in the cochlea or acceleration in the vestibule, results in the deflection of the stereocilia bundle at the apex of the sensory hair cells, which in turn opens the mechanotransduction channels located at the tip of the stereo-cilia3. We sought to elucidate the role of otogelin in the auditory and vestibular functions by generating mice with a targeted disruption of Otog. In Otog−/− mice, both the vestibular and the auditory functions were impaired. Histological analysis of these mutants demonstrated that in the vestibule, otogelin is required for the anchoring of the otoconial membranes and cupulae to the neuroepithelia. In the cochlea, ultrastructural analysis of the TM indicated that otogelin is involved in the organization of its fibrillar network. Otogelin is likely to have a role in the resistance of this membrane to sound stimulation. These results support OTOG as a possible candidate gene for a human nonsyndromic form of deafness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Petit, C. Genes responsible for human hereditary deafness: symphony of a thousand. Nature Genet. 14, 385–391 (1996).
Cohen-Salmon, M., El-Amraoui, A., Leibovici, M. & Petit, C. Otogelin: a glycoprotein specific to the acellular membranes of the inner ear. Proc. Natl Acad. Sci. USA 94, 14450 –14455 (1997).
Hudspeth, A.J. How the ear's works work. Nature 341, 397 –404 (1989).
Ossenkopp, K.P., Prkacin, A. & Hargreaves, E.L. Sodium arsanilate-induced vestibular dysfunction in rats: effects of open-field behavior and spontaneous activity in the automated digiscan monitoring system. Pharmacol. Biochem. Behav. 36, 875–881 (1990).
Porter, J.D., Pellis, S.M. & Meyer, M.E. An open-field activity analysis of labyrinthectomized rats. Physiol. Behav. 48, 27– 30 (1990).
Llorens, J., Dermenes, D. & Sans, A. The behavioral syndrome caused by 3,3′-iminodipropionitrile and related nitriles in the rat is associated with degeneration of the vestibular sensory hair cells. Toxicol. Applied Pharmacol. 123 , 199–210 (1993).
Henry, K.R. Auditory nerve and brain stem volume-conducted potentials by pure-tone pips in the CBA/J laboratory mouse. Audiology 18, 93–108 (1979).
Legan, P.K., Rau, A., Keen, J.N. & Richardson, G.P. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J. Biol. Chem. 272 , 8791–8801 (1997).
Rau, A., Legan, P.K. & Richardson, G.P. Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J. Comp. Neurol. 405, 271–280 ( 1999).
Munyer, P.D. & Schulte, B.A. Immunohistochemical localization of keratan sulfate and chondroitin 4- and 6-sulfate proteoglycans in subregions of the tectorial and basilar membranes. Hearing Res. 79, 83–93 (1994).
Lim, D.J. Fine morphology of the tectorial membrane. Its relationship to the organ of Corti. Arch. Otolaryngol. 96, 199– 215 (1972).
Kronester-Frei, A. Ultrastructure of the different zones of the tectorial membrane. Cell Tissue Res. 193, 11–23 (1978).
Hasko, J.A. & Richardson, G.P. The ultrastructural organization and properties of the mouse tectorial membrane matrix. Hearing Res. 35, 21–38 ( 1988).
Tsuprun, V. & Santi, P. Ultrastructure organization of proteoglycans and fibrillar matrix of the tectorial membrane. Hearing Res. 110, 107–118 (1997).
Slepecky, N.B., Cefaratti, L.K. & Yoo, T.J. Type II and type IX collagen form heterotypic fibers in the tectorial membrane of the inner ear. Matrix 12, 80–86 (1992).
Slepecky, N.B., Savage, S.E., Cerafatti, L.K. & Yoo, T.J. Electron-microscopic localization of type II,IX and V collagen in the organ of Corti. Cell Tissue Res. 267, 413– 418 (1992).
Zwislocki, J.J., Chamberlain, S.C. & Slepecky, N.B. Tectorial membrane. I: static mechanical properties in vivo. Hearing Res. 33, 207– 222 (1988).
Keats, B.J., Noury, N., Pelias, M.Z., Deininger, P.L. & Litt, M. Tightly linked flanking microsatellite markers for the Usher syndrome type I locus on the short arm of chromosome 11. Am. J. Hum. Genet. 54, 681–686 (1994).
Jain, P.K. et al. A gene responsible for nonsyndromic sensorineural deafness (DFNB18) maps to the chromosomal region 11p14–p15.1 containing the Usher syndrome type 1C gene. Genomics 50, 290–292 (1998).
Cohen-Salmon, M., Mattei, M.-G. & Petit, C. Mapping of the otogelin gene (OTGN) to mouse chromosome 7 and human chromosome 11p14.3: a candidate for human autosomal recessive nonsyndromic deafness DFNB18. Mamm. Genome 10, 520–522 (1999).
Kelsell, D.P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80– 83 (1997).
Xia, J.-h. et al. Mutations in the gene encoding gap junction protein ß-3 associated with autosomal dominant hearing impairment. Nature Genet. 20, 370–373 ( 1998).
Kubisch, C. et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96 , 437–446 (1999).
Grifa, A. et al. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nature Genet. 23, 16–18 (1999).
Cohen-Tannoudji, M. et al. Disruption of murine Hexa gene leads to enzymatic deficiency and to neuronal lysosomal storage, similar to that observed in Tay-Sachs disease. Mamm. Genome 6, 844–849 (1995).
Verpy, E., Leibovici, M. & Petit, C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc. Natl Acad. Sci. USA 96, 529– 534 (1999).
Bernex, F. et al. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development 122, 3023–3033 (1996).
Steel, K.P. & Hardisty, R. Assessing hearing, vision and balance in mice. in What's Wrong with My Mouse? New Interplays Between Mouse Genes and Behavior 26–38 (Soc. Neurosci. short course syllabus, Washington DC, 1996).
Zheng, Q.Y., Johnson, K.R. & Reway, L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Res. 130, 94–107 (1999).
Erway, L.C., Willott, J.F., Archer, J.R. & Harrison, D.E. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hearing Res. 65, 125–132 (1993).
Acknowledgements
We thank G. Richardson for his contribution to the analysis of ultrastructural data; C. Elbaz for technical help in histological analysis; C. Koenen for care of the animals; S. Blot for help in ABR recording; R. Hellio for confocal microscopy; O. Ardouin, F. Bernex and U. Rogne for helpful advice; T. White, J.-P. Hardelin and D. Weil for critical reading of the manuscript; and J. Levilliers for constant help and support. This work was supported by the Centre National de la Recherche Scientifique (CNRS; Action Concertée Biologie cellulaire, grant 96114) and by the European Community (grant QLG2-CT 1999-00988). M.-C.S. is CR at the CNRS.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Simmler, MC., Cohen-Salmon, M., El-Amraoui, A. et al. Targeted disruption of Otog results in deafness and severe imbalance . Nat Genet 24, 139–143 (2000). https://doi.org/10.1038/72793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72793
This article is cited by
-
Intragenic homozygous duplication in HEPACAM is associated with megalencephalic leukoencephalopathy with subcortical cysts type 2A
neurogenetics (2024)
-
Types of Inheritance and Genes Associated with Familial Meniere Disease
Journal of the Association for Research in Otolaryngology (2023)
-
Betahistine alleviates benign paroxysmal positional vertigo (BPPV) through inducing production of multiple CTRP family members and activating the ERK1/2-AKT/PPARy pathway
Biological Research (2022)
-
First glance at the molecular etiology of hearing loss in French-Canadian families from Saguenay-Lac-Saint-Jean’s founder population
Human Genetics (2022)
-
Vestibular Drop Attacks and Meniere’s Disease as Results of Otolithic Membrane Damage—A Numerical Model
Journal of the Association for Research in Otolaryngology (2022)