Abstract
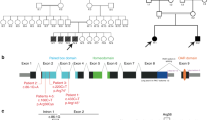
Nemaline myopathies are diseases characterized by the presence in muscle fibres of pathognomonic rod bodies. These are composed largely of α–actinin and actin. We have identified a missense mutation in the α–tropomyosin gene, TPM3, which segregates completely with the disease in a family whose autosomal dominant nemaline myopathy we had previously localized to chromosome 1p13–q25. The mutation substitutes an arginine residue for a highly conserved methionine in a putative actin–binding site near the N terminus of the α–tropomyosin. The mutation may strengthen tropomyosin – actin binding, leading to rod body formation, by adding a further basic residue to the postulated actin–binding motif.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bodensteiner, J.B. Congenital myopathies. Muscle Nerve 17, 131–144 (1994).
Shy, G., Engel, W., Somers, J. & Wanko, T. Nemaline myopathy: a new congenital myopathy. Brain 86, 793–810 (1963).
Conen, P.E., Murphy, E.G. & Donohue, W.L. Light and electron microscopic studies of “myogranules” in a child with hypotonia and muscle weakness. Can. Med. Ass. J. 89, 983–986 (1963).
McKusick, V.A. Mendelian inheritance in man 10th edn (Johns Hopkins University Press, Baltimore and London, 1992).
Laing, N.G. et al. Assignment of a gene (NEM1) for autosomal dominant nemaline myopathy to chromosome 1. Am. J. hum. Genet. 50, 576–583 (1992).
Eyre, H.J. et al. Assignment of the human slow skeletal muscle troponin gene (TNNI1) to 1q32 by fluorescence in situ hybridisation. Cytogenet. Cell Genet. 62, 181–182 (1993).
Akkari, P.A. et al. Assignment of the human skeletal muscle α actin gene (ACTA1) to 1q42 by fluorescence in situ hybridisation. Cytogenet. Cell Genet. 65, 265–267 (1994).
Wilton, S.D. et al. Assignment of the human α tropmyosin gene TPM3 to 1q22–23 by fluorescence in situ hybridisation. Cytogenet Cell Genet. 68, 122–124 (1994).
Gunning, P. et al. Differential control of tropomyosin mRNA levels during myogenesis suggests the existence of an isoform competition-autoregulatory compensation control mechanism. Dev. Biol. 138, 443–453 (1990).
McAlpine, P.J., Shows, T.B., Boucheix, C., Pericak-Vance, M.A. & Anderson, W.A. in Chromosome coordinating meeting (1992). (eds Cuticchia, A.J., Pearson, P.L. & Klinger, H.P.). 11–142 (Karger, Basel, 1993).
Clayton, L., Reinach, F.C., Chumbley, G.M. & MacLeod, A.R. Organization of the hTMnm gene. Implications for the evolution of muscle and non-muscle tropomyosins. J. molec. Biol. 201, 507–515 (1988).
Wolfe, S.L. Molecular and cellular biology. 451–494 (Wadsworth Publishing Company, Belmont, 1993).
Thierfelder, L. et al. α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 77, 701–712 (1994).
Geisterfer-Lowrance, A.A.T. et al. A molecular basis for familial hypertrophic cardiomyopathy: a β cardiac myosin heavy chain gene missense mutation. Cell 62, 999–1006 (1990).
Price, H.M., Gordon, G.B., Pearson, C.M., Munsat, T.L. & Blumberg, J.M. New evidence for excessive accumulation of Z-band material in nemaline myopathy. Proc. natn. Acad. Sci. U.S.A. 54, 1398–1406 (1965).
NIH/CEPH Collaborative Mapping Group. A comprehensive genetic linkage map of the human genome. Science 258, 67–86 (1992).
Gyapay, G. et al. The 1993–94 Généthon human genetic linkage map. Nature Genet. 7, 246–339 (1994).
Ott, J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am. J. hum. Genet. 26, 588–597 (1974).
Orita, M., Suzuki, Y., Sekiya, T. & Hayashi, K. Rapid and sensitive detection of point mutations and DMA polymorphisms using the polymerase chain reaction. Genomics 5, 874–879 (1989).
Tahvanalnen, E., Beggs, A.H. & Wallgren-Pettersson, C. Exclusion of two candidate loci for autosomal recessive nemaline myopathy. J. med. Genet. 31, 79–80 (1994).
Cho, Y.-J., Liu, J. & Hitchcock-DeGregori, S.E. The amino terminus of muscle tropomyosin is a major determinant for function. J. biol. Chem. 265, 538–545 (1990).
Yonezawa, N. et al. An actin-interacting heptapeptide in the cofilin sequence. Eur. J. Biochem. 183, 235–238 (1989).
Hitchcock-DeGregori, S.E. & Vamell, T.A. Tropomyosin has discrete actin-binding sites with sevenfold and fourteenfold periodicities. J. molec. Biol. 214, 885–896 (1990).
Hayasaka, K. et al. Charcot-Marie-Tooth neuropathy type 1B is associated with mutations of the myelin Po gene. Nature Genet. 5, 31–34 (1993).
Eyre, H. et al. Assignment of the human skeletal muscle a tropomyosin gene TPM1 to 15q22 by fluorescence in situ hybridisation. Cytogenet. Cell Genet. (in the press).
Beggs, A.H. et al. A (CA)n repeat polymorphism for the skeletal muscle α-actinin gene ACTN2 and its localisation on the CEPH consortium linkage map of chromosome 1. Genomics 13, 1314–1315 (1992).
Wilton, S.D., Johnsen, R.D., Pedretti, J.R. & Laing, N.G. Two distinct mutations in a single dystrophin gene: identification of an altered splice-site as the primary becker muscular dystrophy mutation. Am. J. med. Genet. 46, 563–569 (1993).
MacLeod, A.R. & Gooding, C. Human hTM-α gene: expression in muscle and non–muscle tissue. Molec. cell. Biol. 8, 433–440 (1988).
Widada, J.S., Ferraz, C., Capony, J.-P. & Liautard, J.-P. Complete nucleotide sequence of the adult human skeletal muscle b-tropomyosin. Nucl. Acids Res. 16, 3109 (1988).
Mak, A.S., Smillie, L.B. & Stewart, G.R. A comparison of the amino acid sequences of rabbit skeletal muscle α- and β-tropomyosins. J. biol. Chem. 255, 3647–3655 (1980).
Ruiz-Opazo, N. & Nadal-Ginard, B. α-tropomyosin gene organization. Alternative splicing of duplicated isotype-specific exons accounts for the production of smooth and striated muscle isoforms. J. biol. Chem. 262, 4755–4765 (1987).
Helfman, D.M., Feramisco, J.R., Ricci, W.M. & Hughes, S.H. Isolation and sequence of a cDNA clone that contains the entire coding region for chicken smooth-muscle a-tropomyosin. J. biol. Chem. 259, 14136–14143 (1984).
Hardy, S., Fiszman, M.Y., Osborne, H.B. & Thiebaud, P. Characterization of muscle and non muscle Xenopus laevis tropomyosin mRNAs transcribed from the same gene. Eur. J. Biochem. 202, 431–440 (1991).
Hardy, S. & Thiebaud, P. Isolation and characterization of cDNA clones encoding the skeletal and smooth muscle Xenopus laevis β tropomyosin isoforms. Biochem. Biophys. Acta 1131, 239–242 (1992).
Meedel, T.H. & Hastings, K.E.M. Striated muscle-type tropomyosin in a chordate smooth muscle, ascidian body-wall muscle. J. biol. Chem. 268, 6755–6764 (1993).
Karlik, C.C., Mahaffey, J.W., Coutu, M.D. & Fyrberg, E.A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell 37, 469–481 (1984).
Baader, C.D., Schmid, V. & Schuchert, P. Characterization of a tropomyosin cDNA from the hydrozoan Podocoryne camea. FEBS Letts. 328, 63–66 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laing, N., Wilton, S., Akkari, P. et al. A mutation in the α tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nat Genet 9, 75–79 (1995). https://doi.org/10.1038/ng0195-75
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0195-75
This article is cited by
-
Clinico-pathological and gene features of 15 nemaline myopathy patients from a single Chinese neuromuscular center
Acta Neurologica Belgica (2024)
-
Case report: cosegregation of a TPM1 in-frame deletion (p.Lys7del) with familial non-compaction cardiomyopathy (NCCM)
Clinical Research in Cardiology (2024)
-
Tropomyosin 3 (TPM3) function in skeletal muscle and in myopathy
Skeletal Muscle (2023)
-
Molecular signatures of inherited and acquired sporadic late onset nemaline myopathies
Acta Neuropathologica Communications (2023)
-
A review of major causative genes in congenital myopathies
Journal of Human Genetics (2023)