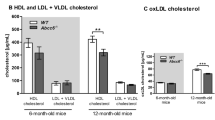
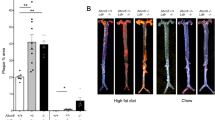
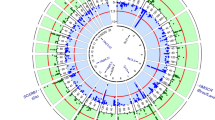
Abstract
The molecular mechanisms regulating the amount of dietary cholesterol retained in the body, as well as the body's ability to exclude selectively other dietary sterols, are poorly understood. An average western diet will contain about 250–500 mg of dietary cholesterol and about 200–400 mg of non-cholesterol sterols. About 50–60% of the dietary cholesterol is absorbed and retained by the normal human body, but less than 1% of the non-cholesterol sterols are retained. Thus, there exists a subtle mechanism that allows the body to distinguish between cholesterol and non-cholesterol sterols. In sitosterolemia, a rare autosomal recessive disorder, affected individuals hyperabsorb not only cholesterol but also all other sterols, including plant and shellfish sterols from the intestine1,2. The major plant sterol species is sitosterol; hence the name of the disorder. Consequently, patients with this disease have very high levels of plant sterols in the plasma and develop tendon and tuberous xanthomas, accelerated atherosclerosis, and premature coronary artery disease3. We previously mapped the STSL locus to human chromosome 2p21 (ref. 4) and further localized it to a region of less than 2 cM bounded by markers D2S2294 and D2S2291 (M.-H.L. et al., manuscript submitted). We now report that a new member of the ABC transporter family, ABCG5, is mutant in nine unrelated sitosterolemia patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bhattacharyya, A.K. & Connor, W.E. β-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J. Clin. Invest. 53, 1033–1043 (1974).
Gregg, R.E., Connor, W.E., Lin, D.S. & Brewer, H. Jr., Abnormal metabolism of shellfish sterols in a patient with sitosterolemia and xanthomatosis. J. Clin. Invest. 77, 1864–1872 (1986).
Bjorkhem, I. & Boberg, K.M. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. in The Metabolic Basis of Inherited Disease (eds. Scriver, C.R., Beaudet, A.L., Sly, W.S. & Valle, D.) 2073–2102 (McGraw-Hill, New York, 1995).
Patel, S.B. et al. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J. Clin. Invest. 102, 1041–1044 (1998).
Shulenin, S. et al. A liver-specific ATP-binding cassette gene (ABCG5) from the ABCG (White) gene subfamily maps to human chromosome 2p21 in the region of the sitosterolemia locus. Cyto. Cell Genet. (in press).
Frischmeyer, P.A. & Dietz, H.C. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 8, 1893–1900 (1999).
Witsch-Baumgartner, M. et al. Mutational spectrum in the δ7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 66, 402–412 (2000).
Brooks-Wilson, A. et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nature Genet. 22, 336–345 (1999).
Bodzioch, M. et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genet. 22, 347–351 (1999).
Rust, S. et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nature Genet. 22, 352–355 (1999).
Erlinger, S. Review article: new insights into the mechanisms of hepatic transport and bile secretion. J. Gastroenterol. Hepatol. 11, 575–579 (1996).
Klein, I., Sarkadi, B. & Varadi, A. An inventory of the human ABC proteins. Biochim. Biophys. Acta 1461, 237–262 (1999).
Klucken, J. et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. USA 97, 817–822 (2000).
Patel, S.B., Honda, A. & Salen, G. Sitosterolemia: exclusion of genes involved in reduced cholesterol biosynthesis. J. Lipid Res. 39, 1055–1061 (1998).
Sossey-Alaoui, K. et al. Molecular cloning and characterization of TRPC5 (HTRP5), the human homologue of a mouse brain receptor-activated capacitative Ca2+ entry channel. Genomics 60, 330–340 (1999).
Yu, H., Tint, G.S., Salen, G. & Patel, S.B. Detection of a common mutation in the RSH or Smith-Lemli-Opitz syndrome by a PCR-RFLP assay: IVS8-G→C is found in over sixty percent of US propositi. Am. J. Med. Genet. 90, 347–350 (2000).
Wu, J., Zhu, Y.H. & Patel, S.B. Cyclosporin-induced dyslipoproteinemia is associated with selective activation of SREBP-2. Am. J. Physiol. 277, E1087–1094 (1999).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Swofford, D.L. & Olsen, G.J. Phylogeny reconstruction. in Molecular Systematics (eds. Hillis, D.M. & Moritz, C.) 411–501 (Sinauer Asociates, Sunderland, 1990).
Felsenstein, J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 266, 418–427 (1996).
Page, R.D. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12, 357–358 (1996).
Berge, K.E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–1775 (2000).
Acknowledgements
We thank the families for participation; Y. Zhou for technical assistance; the General Clinical Research Center, MUSC, for assistance with sequencing; and the members of the Patel lab for discussions. This work was supported by grants from the NIH and USPHS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, MH., Lu, K., Hazard, S. et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet 27, 79–83 (2001). https://doi.org/10.1038/83799
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83799
This article is cited by
-
The TICE Pathway: Mechanisms and Potential Clinical Applications
Current Atherosclerosis Reports (2023)
-
Features of chinese patients with sitosterolemia
Lipids in Health and Disease (2022)
-
Phytosterols and Cardiovascular Disease
Current Atherosclerosis Reports (2021)
-
Interleukin-33 delays recovery of mucosal inflammation via downregulation of homeostatic ABCG5/8 in the colon
Laboratory Investigation (2020)
-
Multi-population GWAS and enrichment analyses reveal novel genomic regions and promising candidate genes underlying bovine milk fatty acid composition
BMC Genomics (2019)