Abstract
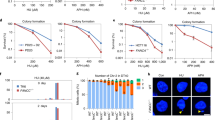
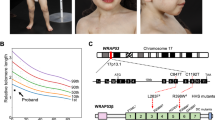
DNA interstrand crosslink repair requires several classes of proteins, including structure-specific endonucleases and Fanconi anemia proteins. SLX4, which coordinates three separate endonucleases, was recently recognized as an important regulator of DNA repair. Here we report the first human individuals found to have biallelic mutations in SLX4. These individuals, who were previously diagnosed as having Fanconi anemia, add SLX4 as an essential component to the FA-BRCA genome maintenance pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
de Winter, J.P. & Joenje, H. The genetic and molecular basis of Fanconi anemia. Mutat. Res. 668, 11–19 (2009).
Vaz, F. et al. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat. Genet. 42, 406–409 (2010).
Singh, T.R. et al. Impaired FANCD2 monoubiquitination and hypersensitivity to camptothecin uniquely characterize Fanconi anemia complementation group M. Blood 114, 174–180 (2009).
Shimamura, A. et al. A novel diagnostic screen for defects in the Fanconi anemia pathway. Blood 100, 4649–4654 (2002).
Fekairi, S. et al. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell 138, 78–89 (2009).
Svendsen, J.M. et al. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell 138, 63–77 (2009).
Andersen, S.L. et al. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structures-specific endonucleases in DNA repair and recombination. Mol. Cell 35, 128–135 (2009).
Muñoz, I.M. et al. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol. Cell 35, 116–127 (2009).
van Vuren, A.J. et al. Evidence for a repair enzyme complex involving ERCC1 and complementing activities of ERCC4, ERCC11 and xeroderma pigmentosum group F. EMBO J. 12, 3693–3701 (1993).
Crossan, G.P. et al. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat. Genet. advance online publication, doi:10.1038/ng.752 (16 January 2011).
Jaspers, N.G.J. et al. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am. J. Hum. Genet. 80, 457–466 (2007).
Ahmad, A. et al. Mislocalization of XPF-ERCC1 nuclease contributes to reduced DNA repair in XP-F patients. PLoS Genet. 6, e1000871 (2010).
Joenje, H. et al. Classification of Fanconi anemia patients by complementation analysis: evidence for a fifth genetic subtype. Blood 86, 2156–2160 (1995).
Acknowledgements
We thank the affected individuals and their families for contributing to this study. We also thank A. Raams, R. Friedl, B. Gottwald and S. Darchinger for expert technical assistance. We acknowledge I. Carr for Phaser software, R. Kanaar for RAD51 antiserum and K.J. Patel for mouse Slx4 cDNA. Financial support was from the Cancer Center Amsterdam-VU Medisch Centrum Institute for Cancer and Immunology (CCA/V-ICI) Amsterdam (to C.S.), the Dutch Cancer Society (to H.J.), Schroeder-Kurth-Fund (to D.S.) and the Medical Research Council UK (to J.R.).
Author information
Authors and Affiliations
Contributions
The study was designed by J.P.d.W., J.R., D.S. and H.J. Clinical information of affected individuals and referral for Fanconi anemia diagnosis was coordinated by E.T.K. and Y.H.-H. Fanconi anemia diagnosis was confirmed by A.W.M.N. SNP array studies were coordinated by T.B. Mutational analysis and functional studies were carried out by C.S., K.H., B.S., M.A.R., J.S., A.B.O. and K.E. The ERCC1 focus formation assay was coordinated by N.G.J.J. The manuscript was written by C.S., J.P.d.W., J.R. and D.S., with help from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–9 (PDF 6260 kb)
Rights and permissions
About this article
Cite this article
Stoepker, C., Hain, K., Schuster, B. et al. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet 43, 138–141 (2011). https://doi.org/10.1038/ng.751
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.751
This article is cited by
-
Pembrolizumab activity in patients with Fanconi anemia repair pathway competent and deficient tumors
Biomarker Research (2022)
-
The structure-specific endonuclease complex SLX4–XPF regulates Tus–Ter-induced homologous recombination
Nature Structural & Molecular Biology (2022)
-
Distinct roles of XPF-ERCC1 and Rad1-Rad10-Saw1 in replication-coupled and uncoupled inter-strand crosslink repair
Nature Communications (2018)
-
SLX4 Prevents GEN1-Dependent DSBs During DNA Replication Arrest Under Pathological Conditions in Human Cells
Scientific Reports (2017)
-
Chronic treatment with cisplatin induces chemoresistance through the TIP60-mediated Fanconi anemia and homologous recombination repair pathways
Scientific Reports (2017)