Abstract
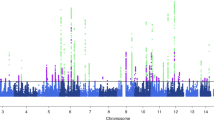
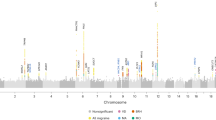
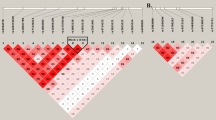
Migraine is a common episodic neurological disorder, typically presenting with recurrent attacks of severe headache and autonomic dysfunction. Apart from rare monogenic subtypes, no genetic or molecular markers for migraine have been convincingly established. We identified the minor allele of rs1835740 on chromosome 8q22.1 to be associated with migraine (P = 5.38 × 10−9, odds ratio = 1.23, 95% CI 1.150–1.324) in a genome-wide association study of 2,731 migraine cases ascertained from three European headache clinics and 10,747 population-matched controls. The association was replicated in 3,202 cases and 40,062 controls for an overall meta-analysis P value of 1.69 × 10−11 (odds ratio = 1.18, 95% CI 1.127–1.244). rs1835740 is located between MTDH (astrocyte elevated gene 1, also known as AEG-1) and PGCP (encoding plasma glutamate carboxypeptidase). In an expression quantitative trait study in lymphoblastoid cell lines, transcript levels of the MTDH were found to have a significant correlation to rs1835740 (P = 3.96 × 10−5, permuted threshold for genome-wide significance 7.7 × 10−5). To our knowledge, our data establish rs1835740 as the first genetic risk factor for migraine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hindorff, L.A., Junkins, H.A., Mehta, J.P. & Manolio, T.A. A catalog of published genome-wide association studies (accessed 16 February 2010). <http://www.genome.gov/gwastudies>.
Hanna, M.G. Genetic neurological channelopathies. Nat. Clin. Pract. Neurol. 2, 252–263 (2006).
Nyholt, D.R. et al. A high-density association screen of 155 ion transport genes for involvement with common migraine. Hum. Mol. Genet. 17, 3318–3331 (2008).
Frankel, W.N. Genetics of complex neurological disease: challenges and opportunities for modeling epilepsy in mice and rats. Trends Genet. 25, 361–367 (2009).
Stovner, L.J., Zwart, J.A., Hagen, K., Terwindt, G.M. & Pascual, J. Epidemiology of headache in Europe. Eur. J. Neurol. 13, 333–345 (2006).
Stovner, L. et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 27, 193–210 (2007).
International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia 24, Suppl 1, 9–160 (2004).
Goadsby, P.J., Lipton, R.B. & Ferrari, M.D. Migraine–current understanding and treatment. N. Engl. J. Med. 346, 257–270 (2002).
Lauritzen, M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 117, 199–210 (1994).
Hadjikhani, N. et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc. Natl. Acad. Sci. USA 98, 4687–4692 (2001).
Frazer, K.A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Nyholt, D.R. et al. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet. 74, 765–769 (2004).
Risch, N.J. Searching for genetic determinants in the new millennium. Nature 405, 847–856 (2000).
Dimas, A.S. et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325, 1246–1250 (2009).
Hu, V.W. et al. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2, 78–97 (2009).
Nishimura, Y. et al. Genome-wide expression profiling of lymphoblastoid cell lines distinguishes different forms of autism and reveals shared pathways. Hum. Mol. Genet. 16, 1682–1698 (2007).
Martin, M.V. et al. Exon expression in lymphoblastoid cell lines from subjects with schizophrenia before and after glucose deprivation. BMC Med. Genomics 2, 62 (2009).
Emdad, L. et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc. Natl. Acad. Sci. USA 106, 21300–21305 (2009).
Kang, D.C. et al. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 353, 8–15 (2005).
Noch, E. & Khalili, K. Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol. Ther. 8, 1791–1797 (2009).
Boycott, H.E., Wilkinson, J.A., Boyle, J.P., Pearson, H.A. & Peers, C. Differential involvement of TNF alpha in hypoxic suppression of astrocyte glutamate transporters. Glia 56, 998–1004 (2008).
Dallas, M. et al. Hypoxia suppresses glutamate transport in astrocytes. J. Neurosci. 27, 3946–3955 (2007).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997).
Goadsby, P.J., Charbit, A.R., Andreou, A.P., Akerman, S. & Holland, P.R. Neurobiology of migraine. Neurosci. 161, 327–341 (2009).
Burstein, R., Cutrer, M.F. & Yarnitsky, D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 123, 1703–1709 (2000).
Ophoff, R.A. et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87, 543–552 (1996).
De Fusco, M. et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 33, 192–196 (2003).
Dichgans, M. et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 366, 371–377 (2005).
Pietrobon, D. Familial hemiplegic migraine. Neurotherapeutics 4, 274–284 (2007).
de Vries, B., Frants, R.R., Ferrari, M.D. & van den Maagdenberg, A.M. Molecular genetics of migraine. Hum. Genet. 126, 115–132 (2009).
Tottene, A. et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 61, 762–773 (2009).
de Vries, B. et al. Episodic ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Arch. Neurol. 66, 97–101 (2009).
Jen, J.C., Wan, J., Palos, T.P., Howard, B.D. & Baloh, R.W. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology 65, 529–534 (2005).
Teo, Y.Y. et al. A genotype calling algorithm for the Illumina BeadArray platform. Bioinformatics 23, 2741–2746 (2007).
Stranger, B.E. et al. Population genomics of human gene expression. Nat. Genet. 39, 1217–1224 (2007).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Gretarsdottir, S. et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat. Genet. 35, 131–138 (2003).
Grant, S.F. et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 38, 320–323 (2006).
Howie, B.N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Acknowledgements
We wish to thank all individuals in the respective cohorts for their generous participation. This work was supported by the Wellcome Trust (grant number WT089062) and, among others, by the Academy of Finland (200923 to AP, 00213 to M.W.); the Helsinki University Central Hospital (to M. Kallela., M.F., V. Artto and S.V.); the Academy of Finland Center of Excellence for Complex Disease Genetics; the EuroHead project (LSM-CT-2004-504837); the Helsinki Biomedical Graduate School (to V. Anttila, P.T.-K.); the Finnish Cultural Foundation (to V. Anttila); the Finnish Neurology Foundation, Biomedicum Helsinki Foundation (to V. Anttila, P.T.-K. and V. Artto); the Cambridge Biomedical Research Centre (to S.C.); the Australian National Health and Medical Research Council Fellowship (339462 and 613674) and the Australian Research Council Future Fellowship (FT0991022) schemes (to D.R.N.); the German Federal Ministry of Education and Research (BMBF) (grant 01GS08121 to M. Dichgans, along with support to H.E.W. in the context of the German National Genome Research Network (NGFN-2 and NGFN-plus) for the Heinz Nixdorf Recall study, and to C.K. (EMINet - 01GS08120) for the National Genome Research Network (Germany; NGFN-1 and NGFN-Plus)); the Center for Molecular Medicine Cologne (to C.K.); the Heinz Nixdorf Foundation for the Heinz Nixdorf Recall study, Deutsche Forschungsgemeinschaft (DFG; to C.K. and H.G.); the Netherlands Organization for the Health Research and Development (ZonMw) no. 90700217 (to G.M.T.) and to the Rotterdam Study (RIDE1 and RIDE2); the Netherlands Organisation for Scientific Research (NWO) VICI (918.56.602) and Spinoza (2009) grants (to M.D.F.); and the Center for Medical Systems Biology (CMSB) established by the Netherlands Genomics Initiative/Netherlands Organisation for Scientific Research (NGI/NWO), project no. 050-060-409 (to C.M.v.D., R.R.F., M.D.F. and A.M.J.M.v.d.M.) and project nos. 050-060-810 and 175.010.2005.011, 911-03-012 (to the Rotterdam Study). We thank the Health 2000 study for providing Finnish control genotypes. The Broad Institute Center for Genotyping and Analysis is supported by a grant from the National Center for Research Resources (US). The KORA research platform was initiated and financed by the Helmholtz Center Munich, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria and is supported within the Munich Center of Health Sciences (MC Health) as part of LMUinnovativ. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. We wish to thank S. Hunt, R. Gwillian, P. Whittaker, S. Potter and A. Tashakkori-Ghanbarian, as well as P. Marin-Garcia, for their invaluable help with this study. Finally, we wish to collectively thank everyone who has contributed to the collection, genotyping and analysis of the individual cohorts.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the current version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The author declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1–4 and Supplementary Note (PDF 726 kb)
Rights and permissions
About this article
Cite this article
the International Headache Genetics Consortium. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet 42, 869–873 (2010). https://doi.org/10.1038/ng.652
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.652
This article is cited by
-
Should migraine without aura be further divided? A study of 1444 female patients with migraine without aura
The Journal of Headache and Pain (2023)
-
Genetics of migraine: where are we now?
The Journal of Headache and Pain (2023)
-
A Meta-Analysis of the Genome-Wide Association Studies on Two Genetically Correlated Phenotypes Suggests Four New Risk Loci for Headaches
Phenomics (2023)
-
Current Update on Categorization of Migraine Subtypes on the Basis of Genetic Variation: a Systematic Review
Molecular Neurobiology (2023)
-
Astrocytic Glutamate Transporters and Migraine
Neurochemical Research (2023)