Abstract
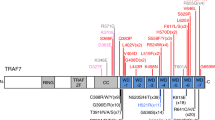
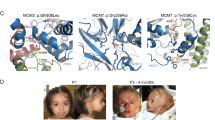
CBL encodes a member of the Cbl family of proteins, which functions as an E3 ubiquitin ligase. We describe a dominant developmental disorder resulting from germline missense CBL mutations, which is characterized by impaired growth, developmental delay, cryptorchidism and a predisposition to juvenile myelomonocytic leukemia (JMML). Some individuals experienced spontaneous regression of their JMML but developed vasculitis later in life. Importantly, JMML specimens from affected children show loss of the normal CBL allele through acquired isodisomy. Consistent with these genetic data, the common p.371Y>H altered Cbl protein induces cytokine-independent growth and constitutive phosphorylation of ERK, AKT and S6 only in hematopoietic cells in which normal Cbl expression is reduced by RNA interference. We conclude that germline CBL mutations have developmental, tumorigenic and functional consequences that resemble disorders that are caused by hyperactive Ras/Raf/MEK/ERK signaling and include neurofibromatosis type 1, Noonan syndrome, Costello syndrome, cardiofaciocutaneous syndrome and Legius syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Niemeyer, C.M. & Kratz, C.P. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia: molecular classification and treatment options. Br. J. Haematol. 140, 610–624 (2008).
Locatelli, F. et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood 105, 410–419 (2005).
Matsuda, K. et al. Spontaneous improvement of hematologic abnormalities in patients having juvenile myelomonocytic leukemia with specific RAS mutations. Blood 109, 5477–5480 (2007).
Flotho, C. et al. Genotype-phenotype correlation in cases of juvenile myelomonocytic leukemia with clonal RAS mutations. Blood 111, 966–967 author reply 967–968 (2008).
Niemeyer, C.M. et al. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS). Blood 89, 3534–3543 (1997).
Kalra, R., Paderanga, D., Olson, K. & Shannon, K.M. Genetic analysis is consistent with the hypothesis that NF1 limits myeloid cell growth through p21ras. Blood 84, 3435–3439 (1994).
Loh, M.L. et al. Somatic mutations in PTPN11 implicate the protein tyrosine phosphatase SHP-2 in leukemogenesis. Blood 103, 2325–2331 (2004).
Shannon, K.M. et al. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N. Engl. J. Med. 330, 597–601 (1994).
Tartaglia, M. et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 34, 148–150 (2003).
Levine, R.L. et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 106, 3377–3379 (2005).
Onida, F. et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 99, 840–849 (2002).
Braun, B.S. et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc. Natl. Acad. Sci. USA 101, 597–602 (2004).
Chan, I.T. et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J. Clin. Invest. 113, 528–538 (2004).
Le, D.T. et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood 103, 4243–4250 (2004).
Mohi, M.G. et al. Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell 7, 179–191 (2005).
Emanuel, P.D., Bates, L.J., Castleberry, R.P., Gualtieri, R.J. & Zuckerman, K.S. Seletive hypersensitivity to granulocyte-macrophage colony stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood 77, 925–929 (1991).
Ramshaw, H.S., Bardy, P.G., Lee, M.A. & Lopez, A.F. Chronic myelomonocytic leukemia requires granulocyte-macrophage colony-stimulating factor for growth in vitro and in vivo. Exp. Hematol. 30, 1124–1131 (2002).
Dunbar, A.J. et al. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 68, 10349–10357 (2008).
Grand, F.H. et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 113, 6181–6192 (2009).
Loh, M.L. et al. Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood 114, 1859–1863 (2009).
Sanada, M. et al. Gain-of-function of mutated C–CBL tumour suppressor in myeloid neoplasms. Nature 460, 904–908 (2009).
Makishima, H. et al. Mutations of e3 ubiquitin ligase cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. J. Clin. Oncol. 27, 6109–6116 (2009).
Bader-Meunier, B. et al. Occurrence of myeloproliferative disorder in patients with the Noonan syndrome. J. Pediatr. 130, 885–889 (1997).
Tartaglia, M. et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am. J. Hum. Genet. 70, 1555–1563 (2002).
Rauen, K.A. et al. Proceedings from the 2009 genetic syndromes of the Ras/MAPK pathway: from bedside to bench and back. Am. J. Med. Genet. A 152A, 4–24 (2009).
Chan, R.J., Cooper, T., Kratz, C.P., Weiss, B. & Loh, M.L. Juvenile myelomonocytic leukemia: A report from the 2nd International JMML Symposium. Leuk. Res. 33, 355–362 (2008).
Hasle, H. et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia 17, 277–282 (2003).
Kotecha, N. et al. Single-cell profiling identifies aberrant stat5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell 14, 335–343 (2008).
Schubbert, S. et al. Functional analysis of leukemia-associated PTPN11 mutations in primary hematopoietic cells. Blood 106, 311–317 (2005).
Langdon, W.Y., Hyland, C.D., Grumont, R.J. & Morse, H.C. III. The c-cbl proto-oncogene is preferentially expressed in thymus and testis tissue and encodes a nuclear protein. J. Virol. 63, 5420–5424 (1989).
Andoniou, C.E., Thien, C.B. & Langdon, W.Y. Tumour induction by activated abl involves tyrosine phosphorylation of the product of the cbl oncogene. EMBO J. 13, 4515–4523 (1994).
Blake, T.J., Shapiro, M., Morse, H.C. III & Langdon, W.Y. The sequences of the human and mouse c–cbl proto-oncogenes show v–cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene 6, 653–657 (1991).
Kassenbrock, C.K. & Anderson, S.M. Regulation of ubiquitin protein ligase activity in c-Cbl by phosphorylation-induced conformational change and constitutive activation by tyrosine to glutamate point mutations. J. Biol. Chem. 279, 28017–28027 (2004).
Thien, C.B. & Langdon, W.Y. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2, 294–307 (2001).
Dittmer, D. et al. Gain of function mutations in p53. Nat. Genet. 4, 42–46 (1993).
Lang, G.A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872 (2004).
Robertson, H., Hime, G.R., Lada, H. & Bowtell, D.D. A Drosophila analogue of v-Cbl is a dominant-negative oncoprotein in vivo. Oncogene 19, 3299–3308 (2000).
Naramura, M. et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 3, 1192–1199 (2002).
Kitaura, Y. et al. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity 26, 567–578 (2007).
Hashimoto, S., Nakano, H., Singh, G. & Katyal, S. Expression of Spred and Sprouty in developing rat lung. Mech. Dev. 119 Suppl 1, S303–S309 (2002).
Oishi, K. et al. Transgenic Drosophila models of Noonan syndrome causing PTPN11 gain-of-function mutations. Hum. Mol. Genet. 15, 543–553 (2006).
Pai, L.M., Barcelo, G. & Schupbach, T. D-cbl, a negative regulator of the Egfr pathway, is required for dorsoventral patterning in Drosophila oogenesis. Cell 103, 51–61 (2000).
The, I. et al. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 276, 791–794 (1997).
Levkowitz, G. et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12, 3663–3674 (1998).
Schubbert, S. et al. Biochemical and functional characterization of germ line KRAS mutations. Mol. Cell. Biol. 27, 7765–7770 (2007).
Mullighan, C.G. et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 106, 9414–9418 (2009).
Wang, X., Huang, D.Y., Huong, S.M. & Huang, E.S. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat. Med. 11, 515–521 (2005).
Ohh, M. et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol. Cell 1, 959–968 (1998).
Ohh, M. et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2, 423–427 (2000).
Acknowledgements
We gratefully acknowledge the generous participation of the families included in this report. Supported in part by the US National Institutes of Health (CA113557 to M.L.L.); the V Foundation for Cancer Research (M.L.L. and B.S.B.); NIH/NCI (K08 CA103868 to B.S.B., R01 CA104282 to M.L.L. and B.S.B.); The Leukemia Lymphoma Society (6059-09, 2157-08 to M.L.L.); the Frank A. Campini Foundation (M.L.L. and B.S.B.); The Concern Foundation (B.S.B.); Deutsche Forschungsgemeinschaft (KR3473/1-1 to C.F.); Deutsche Krebshilfe (108220 to C.M.N. and C.F.); Deutsche José Carreras Leukämie-Stiftung (R08/19 to C.F.); the Canadian Cancer Society (16056 to M.O.); and the National Institute of General Medical Sciences (T32GM007618 to D.H.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
C.M.N. coordinated and collected clinical data from the subjects with EWOG-MDS and wrote the manuscript; M.W.K. collected clinical data, performed laboratory assays including sequencing, proliferation assays and prepared figures; D.H.S. performed laboratory assays including the shRNA experiments, the proliferation assays and protein blots; I.F. collected clinical data; M.E. collected subject samples and performed mutational analysis on highly purified populations of blood cells; N.J.B. contributed subject samples; S.B. performed ubiquitin assays; J.Z.F. and K.M.S. contributed subject samples; T.A.G. performed RNA isolation and cDNA sequencing; P.M. contributed subject samples; I.S., G.K., S.C., P.J.L., C.K. and P.G.S. contributed subject samples; A.H. provided age-matched control samples from children with asthma; M.S. performed mutational analysis; J.S., M.M.v.H., H.H. and F.L. contributed subject samples and collected clinical data; D.S. collected clinical data; S.A. performed colony assays and performed cDNA sequencing; L.C. collected clinical data; R.C.R. and S.S.S. performed ubiquitylation assays; M.O. supervised the ubiquitylation assays and wrote the manuscript; B.S.B. oversaw the shRNA and cell proliferative experiments and wrote the manuscript; C.F. collected clinical data and performed sequencing; M.L.L. coordinated and collected clinical and laboratory data from the USA, oversaw all of the laboratory work, coordinated the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 and Supplementary Note (PDF 2618 kb)
Rights and permissions
About this article
Cite this article
Niemeyer, C., Kang, M., Shin, D. et al. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet 42, 794–800 (2010). https://doi.org/10.1038/ng.641
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.641
This article is cited by
-
Yield of genetic evaluation in non-syndromic pediatric moyamoya patients
Child's Nervous System (2024)
-
CSF1R inhibition for histiocytic neoplasm with CBL mutations refractory to MEK1/2 inhibition
Leukemia (2023)
-
Mining cancer genomes for change-of-metabolic-function mutations
Communications Biology (2023)
-
The International Consensus Classification (ICC) of hematologic neoplasms with germline predisposition, pediatric myelodysplastic syndrome, and juvenile myelomonocytic leukemia
Virchows Archiv (2023)
-
Integrated multi-omics for rapid rare disease diagnosis on a national scale
Nature Medicine (2023)