Abstract
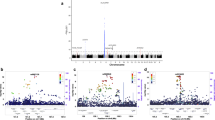
To identify multiple sclerosis (MS) susceptibility loci, we conducted a genome-wide association study (GWAS) in 1,618 cases and used shared data for 3,413 controls. We performed replication in an independent set of 2,256 cases and 2,310 controls, for a total of 3,874 cases and 5,723 controls. We identified risk-associated SNPs on chromosome 12q13–14 (rs703842, P = 5.4 × 10−11; rs10876994, P = 2.7 × 10−10; rs12368653, P = 1.0 × 10−7) and upstream of CD40 on chromosome 20q13 (rs6074022, P = 1.3 × 10−7; rs1569723, P = 2.9 × 10−7). Both loci are also associated with other autoimmune diseases1,2,3,4,5. We also replicated several known MS associations (HLA-DR15, P = 7.0 × 10−184; CD58, P = 9.6 × 10−8; EVI5-RPL5, P = 2.5 × 10−6; IL2RA, P = 7.4 × 10−6; CLEC16A, P = 1.1 × 10−4; IL7R, P = 1.3 × 10−3; TYK2, P = 3.5 × 10−3) and observed a statistical interaction between SNPs in EVI5-RPL5 and HLA-DR15 (P = 0.001).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
24 November 2009
In the version of this article initially published, the 8th SNP in Table 3 was incorrectly listed as rs8118449. The correct identification number for this SNP is rs8112449. The error has been corrected in the HTML and PDF versions of the article.
References
Jacobson, E.M. et al. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun. 8, 205–214 (2007).
Raychaudhuri, S. et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat. Genet. 40, 1216–1223 (2008).
Bailey, R. et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 56, 2616–2621 (2007).
Fung, E. et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 10, 188–191 (2008).
Barton, A. et al. Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat. Genet. 40, 1156–1159 (2008).
Hafler, D.A. et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357, 851–862 (2007).
Rubio, J.P. et al. Replication of KIAA0350, IL2RA, RPL5 and CD58 as multiple sclerosis susceptibility genes in Australians. Genes Immun. 9, 624–630 (2008).
International Multiple Sclerosis Genetics Consortium. The expanding genetic overlap between multiple sclerosis and type I diabetes. Genes Immun. 10, 11–14 (2009).
Aulchenko, Y.S. et al. Genetic variation in the KIF1B locus influences susceptibility to multiple sclerosis. Nat. Genet. 40, 1402–1403 (2008).
Ban, M. et al. Replication analysis identifies TYK2 as a multiple sclerosis susceptibility factor. Eur. J. Hum. Genet. advance online publication, doi: 10.1038/ejhg.2009.41 (18 March 2009).
Adorini, L. & Penna, G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 4, 404–412 (2008).
Chen, S. et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 179, 1634–1647 (2007).
Boonstra, A. et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 167, 4974–4980 (2001).
Penna, G. & Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 164, 2405–2411 (2000).
Lemire, J.M. & Archer, D.C. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J. Clin. Invest. 87, 1103–1107 (1991).
Cantorna, M.T., Hayes, C.E. & DeLuca, H.F. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J. Nutr. 128, 68–72 (1998).
Munger, K.L., Levin, L.I., Hollis, B.W., Howard, N.S. & Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. J. Am. Med. Assoc. 296, 2832–2838 (2006).
Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
van der Mei, I.A. et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J. Neurol. 254, 581–590 (2007).
Ishida, T. et al. CD40 signaling-mediated induction of Bcl-XL, Cdk4, and Cdk6. Implication of their cooperation in selective B cell growth. J. Immunol. 155, 5527–5535 (1995).
Marzo, N. et al. Cyclin-dependent kinase 4 hyperactivity promotes autoreactivity in the immune system but protects pancreatic cell mass from autoimmune destruction in the nonobese diabetic mouse model. J. Immunol. 180, 1189–1198 (2008).
Sekine, C. et al. Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors. J. Immunol. 180, 1954–1961 (2008).
Satoh, J. et al. T cell gene expression profiling identifies distinct subgroups of Japanese multiple sclerosis patients. J. Neuroimmunol. 174, 108–118 (2006).
Durie, F.H., Foy, T.M., Masters, S.R., Laman, J.D. & Noelle, R.J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol. Today 15, 406–411 (1994).
Gerritse, K. et al. CD40–CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 93, 2499–2504 (1996).
Durie, F.H. et al. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science 261, 1328–1330 (1993).
Jacobson, E.M., Concepcion, E., Oashi, T. & Tomer, Y.A. Graves' disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology 146, 2684–2691 (2005).
Barrett, J.C., Fry, B., Maller, J. & Daly, M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Poser, C.M. et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann. Neurol. 13, 227–231 (1983).
McDonald, W.I. et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 50, 121–127 (2001).
Paty, D.W. et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology 38, 180–185 (1988).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Browning, B.L. & Browning, S.R. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am. J. Hum. Genet. 84, 210–223 (2009).
Browning, S.R. & Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097 (2007).
Acknowledgements
We thank individuals with MS in Australia and New Zealand for supporting this research. We are grateful to M. Tanner for database management and J. Wright and C. Remediakis from Multiple Sclerosis Research Australia (MSRA) for expediting this research. J.P.R. and M.B. are supported by Career Development Awards from The National Health and Medical Research Council of Australia (NHMRC). M.A.B. is an NHMRC Principal Research Fellow. H.B. is an NHMRC Peter Doherty Post-doctoral Fellow. J.F. is an MSRA Post-doctoral Fellow. M.B.C. is supported by a grant from the John Hunter Hospital Charitable Trust Fund and a special grant from Macquarie Bank. Replication genotyping was conducted at the Murdoch Children's Research Institute Sequenom Platform Facility. This work was supported by MSRA, John T. Reid Charitable Trusts, Trish MS Research Foundation and the Australian Research Council, under the Linkage Projects Scheme (LP0776744).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Additional information
A complete list of authors and affiliations is provided at the end of the article.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Figures 1–3 and Supplementary Note (PDF 754 kb)
Rights and permissions
About this article
Cite this article
The Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene). Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 41, 824–828 (2009). https://doi.org/10.1038/ng.396
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.396
This article is cited by
-
Epstein–Barr virus and multiple sclerosis
Nature Reviews Microbiology (2023)
-
Immunoglobulin directly enhances differentiation of oligodendrocyte-precursor cells and remyelination
Scientific Reports (2023)
-
The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation
Drugs (2023)
-
The genetics of autoimmune Addison disease: past, present and future
Nature Reviews Endocrinology (2022)
-
Experimental laboratory biomarkers in multiple sclerosis
Wiener Medizinische Wochenschrift (2022)