Abstract
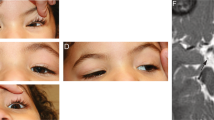
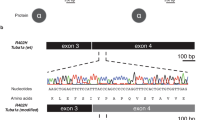
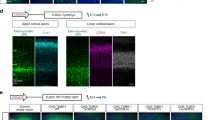
Polymicrogyria is a relatively common but poorly understood defect of cortical development characterized by numerous small gyri and a thick disorganized cortical plate lacking normal lamination. Here we report de novo mutations in a β-tubulin gene, TUBB2B, in four individuals and a 27-gestational-week fetus with bilateral asymmetrical polymicrogyria. Neuropathological examination of the fetus revealed an absence of cortical lamination associated with the presence of ectopic neuronal cells in the white matter and in the leptomeningeal spaces due to breaches in the pial basement membrane. In utero RNAi-based inactivation demonstrates that TUBB2B is required for neuronal migration. We also show that two disease-associated mutations lead to impaired formation of tubulin heterodimers. These observations, together with previous data, show that disruption of microtubule-based processes underlies a large spectrum of neuronal migration disorders that includes not only lissencephaly and pachygyria, but also polymicrogyria malformations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Dutcher, S.K. The tubulin fraternity: alpha to eta. Curr. Opin. Cell Biol. 13, 49–54 (2001).
Keays, D.A. et al. Mutations in α-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell 128, 45–57 (2007).
Poirier, K. et al. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin α 1A (TUBA1A). Hum. Mutat. 28, 1055–1064 (2007).
Bahi-Buisson, N. et al. Refinement of cortical dysgeneses spectrum associated with TUBA1A mutations. J. Med. Genet. 45, 647–653 (2008).
Li, S. et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J. Neurosci. 28, 5817–5826 (2008).
Piao, X. et al. G protein-coupled receptor-dependent development of human frontal cortex. Science 303, 2033–2036 (2004).
Kriegstein, A.R. & Noctor, S.C. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 27, 392–399 (2004).
Marin, O. & Rubenstein, J.L. A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790 (2001).
Metin, C., Baudoin, J.P., Rakic, S. & Parnavelas, J.G. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur. J. Neurosci. 23, 894–900 (2006).
Bai, J. et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat. Neurosci. 6, 1277–1283 (2003).
Nogales, E., Whittaker, M., Milligan, R.A. & Downing, K.H. High-resolution model of the microtubule. Cell 96, 79–88 (1999).
Nogales, E., Wolf, S.G. & Downing, K.H. Structure of the αβ tubulin dimer by electron crystallography. Nature 391, 199–203 (1998).
Lewis, S.A., Tian, G. & Cowan, N.J. The α- and β-tubulin folding pathways. Trends Cell Biol. 7, 479–484 (1997).
Tian, G. et al. Pathway leading to correctly folded β-tubulin. Cell 86, 287–296 (1996).
Cleveland, D.W., Kirschner, M.W. & Cowan, N.J. Isolation of separate mRNAs for α- and β-tubulin and characterization of the corresponding in vitro translation products. Cell 15, 1021–1031 (1978).
Tian, G. et al. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J. Cell Biol. 138, 821–832 (1997).
Little, M. & Seehaus, T. Comparative analysis of tubulin sequences. Comp. Biochem. Physiol. B 90, 655–670 (1988).
Luduena, R.F. Are tubulin isotypes functionally significant. Mol. Biol. Cell 4, 445–457 (1993).
Sullivan, K.F. & Cleveland, D.W. Identification of conserved isotype-defining variable region sequences for four vertebrate β tubulin polypeptide classes. Proc. Natl. Acad. Sci. USA 83, 4327–4331 (1986).
Yu, J.Y., DeRuiter, S.L. & Turner, D.L. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 6047–6052 (2002).
Matsuda, T. & Cepko, C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 101, 16–22 (2004).
Studier, F.W., Rosenberg, A.H., Dunn, J.J. & Dubendorff, J.W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185, 60–89 (1990).
Gao, M. & Knipe, D.M. Distal protein sequences can affect the function of a nuclear localization signal. Mol. Cell. Biol. 12, 1330–1339 (1992).
Tian, G. et al. A pachygyria-causing α-tubulin mutation results in inefficient cycling with CCT and a deficient interaction with TBCB. Mol. Biol. Cell 19, 1152–1161 (2008).
Cowan, N.J. & Lewis, S.A. Type II chaperonins, prefoldin, and the tubulin-specific chaperones. Adv. Protein Chem. 59, 73–104 (2001).
Vainberg, I.E. et al. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell 93, 863–873 (1998).
Spiess, C., Meyer, A.S., Reissmann, S. & Frydman, J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 14, 598–604 (2004).
Tian, G., Vainberg, I.E., Tap, W.D., Lewis, S.A. & Cowan, N.J. Quasi-native chaperonin-bound intermediates in facilitated protein folding. J. Biol. Chem. 270, 23910–23913 (1995).
Acknowledgements
We thank F. Francis for her helpful comments and critical readings of the manuscript, the subjects and their parents who contributed in this study and all the colleagues who provided clinical and imaging information. We thank R. Guerrini for providing us helpful advice. We are grateful to E. Leguern for allowing K.P. to develop this project and all the members of Cochin Institute genomic platform, Cochin Hospital Cell Bank, and Isabelle Souville for their technical assistance. This work was supported by funding from AP-HP, INSERM, FRM (funding within the frame of the Programme EQUIPEs FRM 2007) and ANR (ANR Neuro 2005, project A05183KS and ANR-06-NEURO-008-01 contract number RPV06055ASA). X.H.J. is supported by a PhD fellowship of the Ministère de l'Enseignement Supérieur et de la Recherche, by a grant for mobility from Université Paris Descartes and an EMBO short-term fellowship (ASTF 66.00-2008) for his work in NYU the Medical Center. K.P. is a post-doctoral researcher supported by FRM (Fondation pour la Recherche Medicale).
Author information
Authors and Affiliations
Contributions
J.C. coordinated and instigated the study with D.A.K. and J.F. X.H.J. performed biochemical, cellular and in vivo functional studies. N.B.-B., K.P. and C.F.-B. recruited cases and controls. N.B.-B., C.F.-B., S.O., P.L., M.K., I.S., G.P., P.P. and C.B. helped in collecting patients. K.P., D.A.K. and Y.S. screened the subject DNAs and performed the genetic analyses. L.C.-P. performed all DNA extractions from subject samples and coordinated interaction with clinicians. C.F.-B. performed the neuropathological analyses. G.T. and N.J.C. provided reagents and expertise for the biochemical study; X.P.K. helped compute and analyze the structures; C.C., E.B., P.B. and A.R. provided expertise and technical assistance for in utero RNAi analysis. F.P.-D.-T. and K.P. performed the RNA in situ hybridization analysis. X.H.J. and K.P. drafted the manuscript with the help of N.J.C. and J.C.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Tables 1 and 2, Supplementary Note and Supplementary Methods (PDF 4891 kb)
Rights and permissions
About this article
Cite this article
Jaglin, X., Poirier, K., Saillour, Y. et al. Mutations in the β-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet 41, 746–752 (2009). https://doi.org/10.1038/ng.380
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.380
This article is cited by
-
Novel maternal duplication of 6p22.3-p25.3 with subtelomeric 6p25.3 deletion: new clinical findings and genotype–phenotype correlations
Molecular Cytogenetics (2023)
-
Insights on the Role of α- and β-Tubulin Isotypes in Early Brain Development
Molecular Neurobiology (2023)
-
Complementing the phenotypical spectrum of TUBA1A tubulinopathy and its role in early-onset epilepsies
European Journal of Human Genetics (2022)
-
New perspectives on cytoskeletal dysregulation and mitochondrial mislocalization in amyotrophic lateral sclerosis
Translational Neurodegeneration (2021)
-
TUBB3 Arg262His causes a recognizable syndrome including CFEOM3, facial palsy, joint contractures, and early-onset peripheral neuropathy
Human Genetics (2021)