Abstract
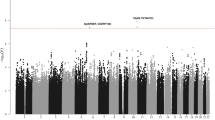
For most immune-mediated diseases, the main determinant of patient well-being is not the diagnosis itself but instead the course that the disease takes over time (prognosis)1,2,3. Prognosis may vary substantially between patients for reasons that are poorly understood. Familial studies support a genetic contribution to prognosis4,5,6, but little evidence has been found for a proposed association between prognosis and the burden of susceptibility variants7,8,9,10,11,12,13. To better characterize how genetic variation influences disease prognosis, we performed a within-cases genome-wide association study in two cohorts of patients with Crohn's disease. We identified four genome-wide significant loci, none of which showed any association with disease susceptibility. Conversely, the aggregated effect of all 170 disease susceptibility loci was not associated with disease prognosis. Together, these data suggest that the genetic contribution to prognosis in Crohn's disease is largely independent of the contribution to disease susceptibility and point to a biology of prognosis that could provide new therapeutic opportunities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Jess, T. et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm. Bowel Dis. 13, 481–489 (2007).
Pincus, T. Long-term outcomes in rheumatoid arthritis. Br. J. Rheumatol. 34 (Suppl. 2), 59–73 (1995).
Weinshenker, B.G. et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112, 133–146 (1989).
Satsangi, J., Grootscholten, C., Holt, H. & Jewell, D.P. Clinical patterns of familial inflammatory bowel disease. Gut 38, 738–741 (1996).
Chataway, J. et al. Multiple sclerosis in sibling pairs: an analysis of 250 families. J. Neurol. Neurosurg. Psychiatry 71, 757–761 (2001).
Jawaheer, D., Lum, R.F., Amos, C.I., Gregersen, P.K. & Criswell, L.A. Clustering of disease features within 512 multicase rheumatoid arthritis families. Arthritis Rheum. 50, 736–741 (2004).
Weersma, R.K. et al. Molecular prediction of disease risk and severity in a large Dutch Crohn's disease cohort. Gut 58, 388–395 (2009).
Hilven, K., Patsopoulos, N.A., Dubois, B. & Goris, A. Burden of risk variants correlates with phenotype of multiple sclerosis. Mult. Scler. 21, 1670–1680 (2015).
Chibnik, L.B. et al. Genetic risk score predicting risk of rheumatoid arthritis phenotypes and age of symptom onset. PLoS One 6, e24380 (2011).
Ananthakrishnan, A.N. et al. Differential effect of genetic burden on disease phenotypes in Crohn's disease and ulcerative colitis: analysis of a North American cohort. Am. J. Gastroenterol. 109, 395–400 (2014).
Jung, C. et al. Genotype/phenotype analyses for 53 Crohn's disease associated genetic polymorphisms. PLoS One 7, e52223 (2012).
Jensen, C.J. et al. Multiple sclerosis susceptibility-associated SNPs do not influence disease severity measures in a cohort of Australian MS patients. PLoS One 5, e10003 (2010).
Scott, I.C. et al. Do genetic susceptibility variants associate with disease severity in early active rheumatoid arthritis? J. Rheumatol. 42, 1131–1140 (2015).
Plomin, R., Haworth, C.M. & Davis, O.S. Common disorders are quantitative traits. Nat. Rev. Genet. 10, 872–878 (2009).
Heliö, T. et al. CARD15/NOD2 gene variants are associated with familially occurring and complicated forms of Crohn's disease. Gut 52, 558–562 (2003).
Cleynen, I. et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet 387, 156–167 (2016).
McKinney, E.F., Lee, J.C., Jayne, D.R., Lyons, P.A. & Smith, K.G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Lee, J.C. et al. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell 155, 57–69 (2013).
Van Gestel, S., Houwing-Duistermaat, J.J., Adolfsson, R., van Duijn, C.M. & Van Broeckhoven, C. Power of selective genotyping in genetic association analyses of quantitative traits. Behav. Genet. 30, 141–146 (2000).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Walter, K. et al. The UK10K project identifies rare variants in health and disease. Nature 526, 82–90 (2015).
Vallot, C. et al. Erosion of X chromosome inactivation in human pluripotent cells initiates with XACT coating and depends on a specific heterochromatin landscape. Cell Stem Cell 16, 533–546 (2015).
Franceschi, C. et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech. Ageing Dev. 126, 351–361 (2005).
Padyukov, L. et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann. Rheum. Dis. 70, 259–265 (2011).
Egea, E. et al. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J. Exp. Med. 173, 531–538 (1991).
Modica, M.A., Cammarata, G. & Caruso, C. HLA-B8,DR3 phenotype and lymphocyte responses to phytohaemagglutinin. J. Immunogenet. 17, 101–107 (1990).
Candore, G. et al. T-cell activation in HLA-B8, DR3-positive individuals. Early antigen expression defect in vitro. Hum. Immunol. 42, 289–294 (1995).
Goyette, P. et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat. Genet. 47, 172–179 (2015).
Sands, B.E. et al. Risk of early surgery for Crohn's disease: implications for early treatment strategies. Am. J. Gastroenterol. 98, 2712–2718 (2003).
Mabbott, N.A., Baillie, J.K., Brown, H., Freeman, T.C. & Hume, D.A. An expression atlas of human primary cells: inference of gene function from coexpression networks. BMC Genomics 14, 632 (2013).
Liu, J.Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Wei, Z. et al. Large sample size, wide variant spectrum, and advanced machine-learning technique boost risk prediction for inflammatory bowel disease. Am. J. Hum. Genet. 92, 1008–1012 (2013).
Anderson, C.A. et al. Data quality control in genetic case–control association studies. Nat. Protoc. 5, 1564–1573 (2010).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Howie, B.N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
O'Connell, J. et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 10, e1004234 (2014).
Delaneau, O., Zagury, J.F. & Marchini, J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods 10, 5–6 (2013).
Liu, J.Z. et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 42, 436–440 (2010).
Zeileis, A., Kleiber, C. & Jackman, S. Regression models for count data in R. J. Stat. Softw. 27, 1–25 (2008).
Cleynen, I. et al. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut 62, 1556–1565 (2013).
Wakefield, J. A Bayesian measure of the probability of false discovery in genetic epidemiology studies. Am. J. Hum. Genet. 81, 208–227 (2007).
Dilthey, A. et al. Multi-population classical HLA type imputation. PLoS Comput. Biol. 9, e1002877 (2013).
Wellek, S. & Ziegler, A. Cochran–Armitage test versus logistic regression in the analysis of genetic association studies. Hum. Hered. 73, 14–17 (2012).
Nielsen, M.M. et al. Identification of expressed and conserved human noncoding RNAs. RNA 20, 236–251 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA–seq aligner. Bioinformatics 29, 15–21 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA–Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Slowikowski, K., Hu, X. & Raychaudhuri, S. SNPsea: an algorithm to identify cell types, tissues and pathways affected by risk loci. Bioinformatics 30, 2496–2497 (2014).
Rossin, E.J. et al. Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet. 7, e1001273 (2011).
Kundu, S., Aulchenko, Y.S., van Duijn, C.M. & Janssens, A.C. PredictABEL: an R package for the assessment of risk prediction models. Eur. J. Epidemiol. 26, 261–264 (2011).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Zheng, J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics http://dx.doi.org/10.1093/bioinformatics/btw613 (2016).
Acknowledgements
We thank L. Hildyard, E. Gray and other members of the Wellcome Trust Sanger Institute DNA team for their help with sample coordination and A. Groff and C. Weiner for critical reading of the manuscript. This work was supported by NIHR Biomedical Research Centres in Cambridge and Guy's and St Thomas' (in particular, J. Todd and the NIHR Cambridge BRC Genomics Theme), Crohn's and Colitis UK (Medical Research Award M/14/2), the Evelyn Trust (17/07), and the Medical Research Council (programme grant MR/L019027/1). J.C.L. is supported by a Wellcome Trust Intermediate Clinical Fellowship (105920/Z/14/Z), and D.B. is supported by a Marie Curie PhD Fellowship (TranSVIR FP7-PEOPLE-ITN-2008 238756). N.J.P. is supported by a Wellcome Trust University Award (094491/Z/10/Z), and J.A.T. is supported by the European Research Council (695551). C.A.A. is supported by the Wellcome Trust (098051). K.G.C.S. is an NIHR Senior Investigator. This study makes use of data generated by the UK10K Consortium, derived from samples from the ALSPAC and DTR cohorts. A full list of the investigators who contributed to the generation of the data is available from http://www.UK10K.org. Funding for UK10K was provided by the Wellcome Trust (WT091310).
Author information
Authors and Affiliations
Consortia
Contributions
The experiment was conceived by J.C.L., M.P., and K.G.C.S. J.C.L., D.B., and P.A.L. designed the analysis. D.B. performed the analysis with input from J.C.L., L.J., C.A.A., J.A.T., and P.A.L. Patient samples and phenotype data were provided by J.C.L., R.R., R.B.G., J.C.M., T.A., N.J.P., J.S., D.C.W., M.P., and other members of the UK IBD Genetics Consortium. J.C.L. and K.G.C.S. wrote the manuscript with input from D.B., P.A.L., and M.P. All authors reviewed and approved the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members and affiliations appears in the Supplementary Note.
Integrated supplementary information
Supplementary Figure 1 Quantile–quantile plot for the combined analysis of cohorts 1 and 2.
Quantile–quantile plot of the observed –log10 (P values) versus the expectation under the null hypothesis. Data are presented for the meta-analysis of cohorts 1 and 2 after imputation and quality control. The overall genomic control inflation factor (λGC) is 1.023, indicating that inflation due to population structure is negligible. SNPs at which the P value is smaller than 1 × 10–8 are represented by triangles at the top of the plot. The gray region represents the 95% concentration band.
Supplementary Figure 2 Fine-mapping at the FOXO3 locus.
Prognosis GWAS results (combined cohorts) at the FOXO3 locus; adapted from the LocusTrack plot1. Top, SNPs in the region with their –log10 (P value) plotted against genomic position and colored according to LD with the lead SNP (rs147856773). Genes in the region are indicated. The expanded plot includes SNPs, genes, and ChIP–seq data from the ENCODE Project2. H3K4me1 and H3K27ac data from CD14+ monocytes and p300 binding data from myeloid K562 cells are shown (no monocyte data were available). The transcription factor binding track displays regions of transcription factor binding identified in a large collection of ChIP–seq experiments performed by the ENCODE Project (further details available at http://genome.ucsc.edu/cgi-bin/hgTrackUi?g=wgEncodeRegTfbsClusteredV3#TRACK_HTML).
Supplementary Figure 3 Transcription of XACT in a range of human tissues.
RNA sequencing data from the XACT locus in a range of human tissues. Raw data were downloaded and aligned against the hg19 genome using Star3. (a–d) The data sets comprised GEO series GSE45326 (ref. 4; n = 1 per tissue) (a,b) and the Illumina Human Bodythe Map 2.0 project (ArrayExpress E-MTAB-513, n = 1 per tissue) (c,d). The bar plots in a and c depict FPKM for the human tissues studied. The tables in b and d contain the raw and normalized data for each tissue.
Supplementary Figure 4 Relationship between association at classical HLA alleles and the frequency with which these alleles occur in non-ancestral MHC 8.1 haplotypes.
Linear regression demonstrating the relationship between the classical HLA alleles that were associated with prognosis and the frequency with which they occur in haplotypes other than the ancestral MHC 8.1 haplotype in Caucasians. Allele frequency and haplotype data were obtained from the National Bone Marrow Donor Program (Six-Locus High Resolution HLA A∼C∼B∼DRB3/4/5∼DRB1∼DQB1 Haplotype Frequencies). Data were not available for HLA-DQA1. In our data, the frequency with which the lead SNP (rs9279411) was associated with non-AH8.1 haplotypes was 0.0149, suggesting that rs9279411 is a better tag for AH8.1 than any of its constituent HLA alleles (and explaining the difference in P values between the HLA alleles and the SNP association).
Supplementary Figure 5 The genetic association signals for Crohn’s disease prognosis and susceptibility at the MHC region are distinct.
(a) Manhattan plots for 22,125 MHC SNPs that were common to this analysis of Crohn’s disease prognosis (top; blue) and a large recent meta-analysis of Crohn’s disease susceptibility (5,956 cases, 14,927 controls5; bottom; red). (b) Scatterplot directly comparing the association P values between Crohn’s disease susceptibility and prognosis at these 22,125 common SNPs. Dotted lines indicate the significance threshold for suggestive association (P < 1 × 10–5). No SNPs that showed suggestive association in one analysis (of susceptibility or prognosis) were also suggestively associated in the other.
Supplementary Figure 6 Protein–protein interaction analysis of genes implicated at prognosis-associated loci.
DAPPLE analysis of prognosis-associated SNPs (meta P < 1 × 10–4) demonstrating known interactions between proteins at implicated loci. Colored dots represent genes at prognosis-associated loci. Gray dots represent proteins at other non-associated loci. Gray lines represent known interactions.
Supplementary Figure 7 Relationship between the observed P value and power for each of the 170 Crohn’s disease susceptibility variants.
Scatterplot of the statistical power to detect a weak general effect (OR = 1.25) plotted against the observed P value in the prognosis analysis for each of the 170 Crohn’s disease susceptibility variants. The line of best fit (dotted line) was calculated by linear regression. Lack of correlation between power and P value is consistent with the null hypothesis that none of the disease susceptibility variants are individually associated with prognosis.
Supplementary Figure 8 Genetic risk scores using the extended Crohn’s disease SNP list (P < 1 × 10–4).
(a–c) Box-and-whisker plots of weighted genetic risk scores between good- and poor-prognosis Crohn’s disease subgroups. (a) L1 (ileal disease, n = 742). (b) L2 (colonic disease, n = 724). (c) L3 (ileocolonic disease, n = 947). Boxes represent the mean and interquartile range. Whiskers represent maximum and minimum values. Genetic risk scores were calculated using an extended list of Crohn’s disease–associated SNPs (P < 1 × 10–4) and their published β values6. (d) Distribution of unweighted risk allele counts in the extended list of Crohn’s disease SNPs between the good-prognosis and poor-prognosis Crohn’s disease subgroups. Purple histogram bars represent the poor-prognosis Crohn’s disease subgroup, and yellow histogram bars represent the good-prognosis Crohn’s disease subgroup. Statistical significance was assessed using unpaired two-tailed Student's t tests and were stratified for disease location; n = 2,413.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–6 and 9, and Supplementary Note (PDF 2834 kb)
Supplementary Table 7
SNPsea results for enrichment of prognosis-associated genes in known biological pathways (Gene Ontology). (XLSX 100 kb)
Supplementary Table 8
SNPsea results for enrichment of prognosis-associated genes in primary human cell types. (XLSX 22 kb)
Supplementary Table 10
Association statistics for 170 Crohn's disease susceptibility SNPs in GWAS of prognosis. (XLSX 30 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Biasci, D., Roberts, R. et al. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn's disease. Nat Genet 49, 262–268 (2017). https://doi.org/10.1038/ng.3755
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3755
This article is cited by
-
DNA methylation fine-tunes pro-and anti-inflammatory signalling pathways in inactive ulcerative colitis tissue biopsies
Scientific Reports (2024)
-
The relationship between extreme inter-individual variation in macrophage gene expression and genetic susceptibility to inflammatory bowel disease
Human Genetics (2024)
-
Global evolving patterns and cross-country inequalities of inflammatory bowel disease burden from 1990 to 2019: a worldwide report
Inflammation Research (2024)
-
A regulatory variant at 19p13.3 is associated with primary biliary cholangitis risk and ARID3A expression
Nature Communications (2023)
-
Ubc9 regulates the expression of MHC II in dendritic cells to enhance DSS-induced colitis by mediating RBPJ SUMOylation
Cell Death & Disease (2023)