Abstract
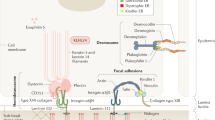
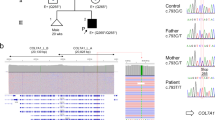
Skin integrity is essential for protection from external stress and trauma. Defects in structural proteins such as keratins cause skin fragility, epitomized by epidermolysis bullosa (EB), a life-threatening disorder. Here we show that dominant mutations of KLHL24, encoding a cullin 3–RBX1 ubiquitin ligase substrate receptor, cause EB. We have identified start-codon mutations in the KLHL24 gene in five patients with EB. These mutations lead to truncated KLHL24 protein lacking the initial 28 amino acids (KLHL24-ΔN28). KLHL24-ΔN28 is more stable than its wild-type counterpart owing to abolished autoubiquitination. We have further identified keratin 14 (KRT14) as a KLHL24 substrate and found that KLHL24-ΔN28 induces excessive ubiquitination and degradation of KRT14. Using a knock-in mouse model, we have confirmed that the Klhl24 mutations lead to stabilized Klhl24-ΔN28 and cause Krt14 degradation. Our findings identify a new disease-causing mechanism due to dysregulation of autoubiquitination and open new avenues for the treatment of related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Uitto, J., Richard, G. & McGrath, J.A. Diseases of epidermal keratins and their linker proteins. Exp. Cell Res. 313, 1995–2009 (2007).
Fine, J.-D. et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J. Am. Acad. Dermatol. 70, 1103–1126 (2014).
Has, C. & Bruckner-Tuderman, L. The genetics of skin fragility. Annu. Rev. Genomics Hum. Genet. 15, 245–268 (2014).
Bruckner-Tuderman, L. & Has, C. Molecular heterogeneity of blistering disorders: the paradigm of epidermolysis bullosa. J. Invest. Dermatol. 132, E2–E5 (2012).
Coulombe, P.A. & Lee, C.H. Defining keratin protein function in skin epithelia: epidermolysis bullosa simplex and its aftermath. J. Invest. Dermatol. 132, 763–775 (2012).
Bonifas, J.M., Rothman, A.L. & Epstein, E.H. Jr. Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science 254, 1202–1205 (1991).
Coulombe, P.A. et al. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell 66, 1301–1311 (1991).
Lane, E.B. et al. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature 356, 244–246 (1992).
McLean, W.H. et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 10, 1724–1735 (1996).
Smith, F.J. et al. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat. Genet. 13, 450–457 (1996).
Koss-Harnes, D. et al. A site-specific plectin mutation causes dominant epidermolysis bullosa simplex Ogna: two identical de novo mutations. J. Invest. Dermatol. 118, 87–93 (2002).
Groves, R.W. et al. A homozygous nonsense mutation within the dystonin gene coding for the coiled-coil domain of the epithelial isoform of BPAG1 underlies a new subtype of autosomal recessive epidermolysis bullosa simplex. J. Invest. Dermatol. 130, 1551–1557 (2010).
Rugg, E.L. et al. Epidermolysis bullosa simplex in Scotland caused by a spectrum of keratin mutations. J. Invest. Dermatol. 127, 574–580 (2007).
Genschik, P., Sumara, I. & Lechner, E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications. EMBO J. 32, 2307–2320 (2013).
Pan, X., Hobbs, R.P. & Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 25, 47–56 (2013).
Toivola, D.M., Boor, P., Alam, C. & Strnad, P. Keratins in health and disease. Curr. Opin. Cell Biol. 32, 73–81 (2015).
Ku, N.O. & Omary, M.B. Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J. Cell Biol. 149, 547–552 (2000).
Rogel, M.R., Jaitovich, A. & Ridge, K.M. The role of the ubiquitin proteasome pathway in keratin intermediate filament protein degradation. Proc. Am. Thorac. Soc. 7, 71–76 (2010).
Yamazaki, S., Uchiumi, A. & Katagata, Y. Hsp40 regulates the amount of keratin proteins via ubiquitin–proteasome pathway in cultured human cells. Int. J. Mol. Med. 29, 165–168 (2012).
Dhanoa, B.S., Cogliati, T., Satish, A.G., Bruford, E.A. & Friedman, J.S. Update on the Kelch-like (KLHL) gene family. Hum. Genomics 7, 13 (2013).
Perez-Torrado, R., Yamada, D. & Defossez, P.A. Born to bind: the BTB protein–protein interaction domain. BioEssays 28, 1194–1202 (2006).
Stogios, P.J. & Privé, G.G. The BACK domain in BTB-kelch proteins. Trends Biochem. Sci. 29, 634–637 (2004).
Bennett, E.J., Rush, J., Gygi, S.P. & Harper, J.W. Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell 143, 951–965 (2010).
Canning, P. et al. Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J. Biol. Chem. 288, 7803–7814 (2013).
Laezza, F. et al. KRIP6: a novel BTB/kelch protein regulating function of kainate receptors. Mol. Cell. Neurosci. 34, 539–550 (2007).
Laezza, F., Wilding, T.J., Sequeira, S., Craig, A.M. & Huettner, J.E. The BTB/kelch protein, KRIP6, modulates the interaction of PICK1 with GluR6 kainate receptors. Neuropharmacology 55, 1131–1139 (2008).
Wu, W. et al. A microRNA encoded by HSV-1 inhibits a cellular transcriptional repressor of viral immediate early and early genes. Sci. China Life Sci. 56, 373–383 (2013).
Weissman, A.M., Shabek, N. & Ciechanover, A. The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 12, 605–620 (2011).
Kigoshi, Y., Tsuruta, F. & Chiba, T. Ubiquitin ligase activity of Cul3-KLHL7 protein is attenuated by autosomal dominant retinitis pigmentosa causative mutation. J. Biol. Chem. 286, 33613–33621 (2011).
Zhuang, M. et al. Structures of SPOP–substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol. Cell 36, 39–50 (2009).
Harper, J.W. & Tan, M.K. Understanding cullin-RING E3 biology through proteomics-based substrate identification. Mol. Cell. Proteomics 11, 1541–1550 (2012).
Schumacher, F.R., Sorrell, F.J., Alessi, D.R., Bullock, A.N. & Kurz, T. Structural and biochemical characterization of the KLHL3–WNK kinase interaction important in blood pressure regulation. Biochem. J. 460, 237–246 (2014).
Ramms, L. et al. Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. USA 110, 18513–18518 (2013).
Gadd, M.S., Bulatov, E. & Ciulli, A. Serendipitous SAD solution for DMSO-soaked SOCS2–elonginC–elonginB crystals using covalently incorporated dimethylarsenic: insights into substrate receptor conformational flexibility in cullin RING ligases. PLoS One 10, e0131218 (2015).
Bikle, D.D., Xie, Z. & Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 7, 461–472 (2012).
Rosenbluth, J.M., Mays, D.J., Pino, M.F., Tang, L.J. & Pietenpol, J.A. A gene signature–based approach identifies mTOR as a regulator of p73. Mol. Cell. Biol. 28, 5951–5964 (2008).
Raj, A., van den Bogaard, P., Rifkin, S.A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Chen, K.H., Boettiger, A.N., Moffitt, J.R., Wang, S. & Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Lloyd, C. et al. The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J. Cell Biol. 129, 1329–1344 (1995).
Löffek, S. et al. The ubiquitin ligase CHIP/STUB1 targets mutant keratins for degradation. Hum. Mutat. 31, 466–476 (2010).
Reichelt, J., Büssow, H., Grund, C. & Magin, T.M. Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol. Biol. Cell 12, 1557–1568 (2001).
Arin, M.J. & Roop, D.R. Disease model: heritable skin blistering. Trends Mol. Med. 7, 422–424 (2001).
Richardson, P.G. et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 348, 2609–2617 (2003).
Kuhn, D.J. et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin–proteasome pathway, against preclinical models of multiple myeloma. Blood 110, 3281–3290 (2007).
Szeverenyi, I. et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 29, 351–360 (2008).
Kumar, P., Henikoff, S. & Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Schwarz, J.M., Rödelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Cooper, G.M. et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 15, 901–913 (2005).
Lichti, U., Anders, J. & Yuspa, S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 3, 799–810 (2008).
Acknowledgements
We thank J. Jin, G. Ou, L. Yu, G. Xu and N. Zheng for advice and support, A. Elia, M. Emanuele and S. Elledge for critical reading of the manuscript, and all the patients and their families for participating in this study. This work was supported by the National Natural Science Foundation of China (grants 81201220 to Z.L., 81271744 to Y.Y. and 31670777 to X.T.), the Young Thousand Talents Program of China to X.T., China National Funds for Distinguished Young Scientists (grant 81425020 to Y.Y.), China National Funds for Excellent Young Scientists (grant 81522037 to Z.L.) and the Beijing Nova Program (grant Z151100000315044 to Z.L.).
Author information
Authors and Affiliations
Contributions
Z.L., S.L., C.F., S.Y., H.W., D.M., Jing Zhang, M.G., X.K., D.B., T.Z., Y.R., X.W. and Y.H. performed the experiments under the supervision of X.T. and Y.Y. O.S., E.S., F.L., T.C., G.P.-B. and D.V. contributed critical reagents. L.D., H.L. and Jianguo Zhang performed the exome sequencing and data analysis. H.D. and F.Z. contributed to the proteomics data analysis. X.T., C.F., S.L., Z.L. and Y.Y. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17 and Supplementary Tables 1–3. (PDF 1846 kb)
Supplementary Table 4
Sequences of primers, sgRNAs, siRNAs and probes used in the study. (XLSX 39 kb)
Rights and permissions
About this article
Cite this article
Lin, Z., Li, S., Feng, C. et al. Stabilizing mutations of KLHL24 ubiquitin ligase cause loss of keratin 14 and human skin fragility. Nat Genet 48, 1508–1516 (2016). https://doi.org/10.1038/ng.3701
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3701
This article is cited by
-
Genome-wide association study identifies genetic variants underlying footrot in Portuguese Merino sheep
BMC Genomics (2024)
-
First case report of complete paternal isodisomy of chromosome 10 harbouring a novel variant in COL17A1 that causes junctional epidermolysis bullosa intermediate
BMC Medical Genomics (2022)
-
Upregulation of KLHL17 promotes the proliferation and migration of non-small cell lung cancer by activating the Ras/MAPK signaling pathway
Laboratory Investigation (2022)
-
Minor hypertrophic cardiomyopathy genes, major insights into the genetics of cardiomyopathies
Nature Reviews Cardiology (2022)
-
AAV-Mediated Artificial miRNA Reduces Pathogenic Polyglucosan Bodies and Neuroinflammation in Adult Polyglucosan Body and Lafora Disease Mouse Models
Neurotherapeutics (2022)