Abstract
SNPs associated with disease susceptibility often reside in enhancer clusters, or super-enhancers. Constituents of these enhancer clusters cooperate to regulate target genes and often extend beyond the linkage disequilibrium (LD) blocks containing risk SNPs identified in genome-wide association studies (GWAS). We identified 'outside variants', defined as SNPs in weak LD with GWAS risk SNPs that physically interact with risk SNPs as part of a target gene's regulatory circuitry. These outside variants further explain variation in target gene expression beyond that explained by GWAS-associated SNPs. Additionally, the clinical risk associated with GWAS SNPs is considerably modified by the genotype of outside variants. Collectively, these findings suggest a potential model in which outside variants and GWAS SNPs that physically interact in 3D chromatin collude to influence target transcript levels as well as clinical risk. This model offers an additional hypothesis for the source of missing heritability for complex traits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maurano, M.T. et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195 (2012).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Trynka, G. et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat. Genet. 45, 124–130 (2013).
Akhtar-Zaidi, B. et al. Epigenomic enhancer profiling defines a signature of colon cancer. Science 336, 736–739 (2012).
Corradin, O. et al. Combinatorial effects of multiple enhancer variants in linkage disequilibrium dictate levels of gene expression to confer susceptibility to common traits. Genome Res. 24, 1–13 (2014).
Farh, K.K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–543 (2015).
Gusev, A. et al. Partitioning heritability of regulatory and cell-type-specific variants across 11 common diseases. Am. J. Hum. Genet. 95, 535–552 (2014).
Whyte, W.A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Parker, S.C. et al. Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc. Natl. Acad. Sci. USA 110, 17921–17926 (2013).
Pasquali, L. et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 46, 136–143 (2014).
Biancolella, M. et al. Identification and characterization of functional risk variants for colorectal cancer mapping to chromosome 11q23.1. Hum. Mol. Genet. 23, 2198–2209 (2014).
Fortini, B.K. et al. Multiple functional risk variants in a SMAD7 enhancer implicate a colorectal cancer risk haplotype. PLoS One 9, e111914 (2014).
Glubb, D.M. et al. Fine-scale mapping of the 5q11.2 breast cancer locus reveals at least three independent risk variants regulating MAP3K1. Am. J. Hum. Genet. 96, 5–20 (2015).
Guo, C. et al. Coordinated regulatory variation associated with gestational hyperglycaemia regulates expression of the novel hexokinase HKDC1. Nat. Commun. 6, 6069 (2015).
He, H. et al. Multiple functional variants in long-range enhancer elements contribute to the risk of SNP rs965513 in thyroid cancer. Proc. Natl. Acad. Sci. USA 112, 6128–6133 (2015).
Roman, T.S. et al. Multiple hepatic regulatory variants at the GALNT2 GWAS locus associated with high-density lipoprotein cholesterol. Am. J. Hum. Genet. 97, 801–815 (2015).
Shaw, A.D. et al. Characterisation of genetic variation in ST8SIA2 and its interaction region in NCAM1 in patients with bipolar disorder. PLoS One 9, e92556 (2014).
Albert, F.W. & Kruglyak, L. The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 16, 197–212 (2015).
Edwards, S.L., Beesley, J., French, J.D. & Dunning, A.M. Beyond GWASs: illuminating the dark road from association to function. Am. J. Hum. Genet. 93, 779–797 (2013).
Nicolae, D.L. et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 6, e1000888 (2010).
Dimas, A.S. et al. Common regulatory variation impacts gene expression in a cell type–dependent manner. Science 325, 1246–1250 (2009).
Wright, F.A. et al. Heritability and genomics of gene expression in peripheral blood. Nat. Genet. 46, 430–437 (2014).
Bryois, J. et al. Cis and trans effects of human genomic variants on gene expression. PLoS Genet. 10, e1004461 (2014).
Ing-Simmons, E. et al. Spatial enhancer clustering and regulation of enhancer-proximal genes by cohesin. Genome Res. 25, 504–513 (2015).
Dowen, J.M. et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159, 374–387 (2014).
Lam, D.D. et al. Partially redundant enhancers cooperatively maintain mammalian Pomc expression above a critical functional threshold. PLoS Genet. 11, e1004935 (2015).
Bothma, J.P. et al. Enhancer additivity and non-additivity are determined by enhancer strength in the Drosophila embryo. eLife 4, e07956 (2015).
Perry, M.W., Boettiger, A.N. & Levine, M. Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 108, 13570–13575 (2011).
Wiersma, E.J., Ronai, D., Berru, M., Tsui, F.W. & Shulman, M.J. Role of the intronic elements in the endogenous immunoglobulin heavy chain locus. Either the matrix attachment regions or the core enhancer is sufficient to maintain expression. J. Biol. Chem. 274, 4858–4862 (1999).
Jeong, Y., El-Jaick, K., Roessler, E., Muenke, M. & Epstein, D.J. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 133, 761–772 (2006).
Montavon, T. et al. A regulatory archipelago controls Hox genes transcription in digits. Cell 147, 1132–1145 (2011).
Perry, M.W., Boettiger, A.N., Bothma, J.P. & Levine, M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr. Biol. 20, 1562–1567 (2010).
Lappalainen, T. et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501, 506–511 (2013).
Mócsai, A., Ruland, J. & Tybulewicz, V.L. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 (2010).
Stranger, B.E. et al. Genome-wide associations of gene expression variation in humans. PLoS Genet. 1, e78 (2005).
Storey, J.D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100, 9440–9445 (2003).
Kasowski, M. et al. Extensive variation in chromatin states across humans. Science 342, 750–752 (2013).
Kilpinen, H. et al. Coordinated effects of sequence variation on DNA binding, chromatin structure, and transcription. Science 342, 744–747 (2013).
Degner, J.F. et al. DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482, 390–394 (2012).
Wang, J. et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 22, 1798–1812 (2012).
Puig-Kröger, A. & Corbí, A. RUNX3: a new player in myeloid gene expression and immune response. J. Cell. Biochem. 98, 744–756 (2006).
Busslinger, M. Transcriptional control of early B cell development. Annu. Rev. Immunol. 22, 55–79 (2004).
Carotta, S., Wu, L. & Nutt, S.L. Surprising new roles for PU.1 in the adaptive immune response. Immunol. Rev. 238, 63–75 (2010).
Sawcer, S. et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
Barrett, J.C. et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 41, 1330–1334 (2009).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Plenge, R.M. et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat. Genet. 39, 1477–1482 (2007).
Cotsapas, C. et al. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 7, e1002254 (2011).
Fortune, M.D. et al. Statistical colocalization of genetic risk variants for related autoimmune diseases in the context of common controls. Nat. Genet. 47, 839–846 (2015).
Gusev, A. et al. Quantifying missing heritability at known GWAS loci. PLoS Genet. 9, e1003993 (2013).
Hemani, G. et al. Detection and replication of epistasis influencing transcription in humans. Nature 508, 249–253 (2014).
Wood, A.R. et al. Another explanation for apparent epistasis. Nature 514, E3–E5 (2014).
Zaitlen, N. & Eskin, E. Imputation aware meta-analysis of genome-wide association studies. Genet. Epidemiol. 34, 537–542 (2010).
Marchini, J. & Howie, B. Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 11, 499–511 (2010).
Guenther, C.A., Tasic, B., Luo, L., Bedell, M.A. & Kingsley, D.M. A molecular basis for classic blond hair color in Europeans. Nat. Genet. 46, 748–752 (2014).
Cowper-Sal·lari, R. et al. Breast cancer risk-associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Nat. Genet. 44, 1191–1198 (2012).
Alcina, A. et al. Identification of a functional variant in the KIF5A–CYP27B1–METTL1–FAM119B locus associated with multiple sclerosis. J. Med. Genet. 50, 25–33 (2013).
Gaulton, K.J. et al. A map of open chromatin in human pancreatic islets. Nat. Genet. 42, 255–259 (2010).
Spieler, D. et al. Restless legs syndrome–associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. 24, 592–603 (2014).
Stadhouders, R. et al. HBS1L–MYB intergenic variants modulate fetal hemoglobin via long-range MYB enhancers. J. Clin. Invest. 124, 1699–1710 (2014).
Selvaraj, S., R Dixon, J., Bansal, V. & Ren, B. Whole-genome haplotype reconstruction using proximity-ligation and shotgun sequencing. Nat. Biotechnol. 31, 1111–1118 (2013).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Howie, B.N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Howie, B., Marchini, J. & Stephens, M. Genotype imputation with thousands of genomes. G3 (Bethesda) 1, 457–470 (2011).
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Yang, J., Lee, S.H., Goddard, M.E. & Visscher, P.M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Acknowledgements
We thank P. Tesar, T. LaFramboise, A. Lager and J. Morrow for helpful comments and discussion. This research was supported by NIH grants R01CA160356, R01CA143237, 1R01CA193677 and P50CA150964 (P.C.S.) and T32GM008056 (O.C.).
Author information
Authors and Affiliations
Contributions
O.C. and P.C.S. designed the study, performed the analyses and interpreted the results. A.J.C. and F.R.S. aided statistical analyses and discussion. O.C., J.M.L. and I.M.B. performed cell culture and luciferase experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Prevalence of outside variants.
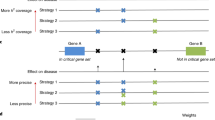
(a) Schematic depicting the outside variant model. Gray represents enhancers that regulate a common gene (blue arrow). SNPs in LD within a GWAS-associated locus are defined as ‘linked variants’ (blue). Additional variants located in putative enhancer elements that regulate the same gene but are not in LD with the linked SNPs are defined as ‘outside variants’ (green). (b,c) The proportion of GWAS loci that contain outside variants is defined by each D′ (b) and r2 (c) threshold.
Supplementary Figure 2 Definition of outside variants used in transcriptome analysis.
(a) Methodology used to define the outside variants that were evaluated in transcriptome analysis. (b) Number of lead SNPs, gene associations, outside variants and GWAS SNP–outside variant pairs that resulted from the methodology described in a. (b–e) For all outside variant–GWAS SNP pairs evaluated in Figure 2, the distribution of r2 (c), D′ (d) and LOD (e) is shown. (f) The minor allele frequency of each outside variant in the 373-individual panel used for transcriptome analysis. (g) Proportion of GWAS loci (total n = 186) that harbor outside variants determined to alter the impact of the GWAS allele on target transcript levels for multiple methods of multiple-test correction.
Supplementary Figure 3 Functional outside variants as compared to published eQTL results.
Proportion of outside variants that have previously been reported as eQTLs (red).
Supplementary Figure 4 Outside variant loci evaluated in luciferase reporter assays.
Sanger sequencing of outside variant genotype for the reporter constructs used in Figure 3g.
Supplementary Figure 5 Heritability explained by local controls as compared to outside variants.
Heritability estimates for ulcerative colitis (left) and multiple sclerosis (right) as compared to 1,000 sets of randomly selected local control variants, variants within 200 kb of a GWAS SNP that do not lie in the regulatory circuitry of the gene target (gray box plot, 5th- to 95th-percentile whiskers; two-sample z test: UC, P = 0.004; MS, P < 0.001).
Supplementary Figure 6 Evaluation of potential third variants for genes with functional outside variants (FDR q < 0.05).
Analytical design and results of the impact of ‘third’ variants on gene expression and clinical risk for all genes with functional outside variants defined at FDR q < 0.05. Dot plots provide examples of outside variant and GWAS alleles that explain additional variation in gene expression.
Supplementary Figure 7 Evaluation of potential third variants for genes with functional variants (FDR q < 0.1 or permutation P < 0.01).
Same as Supplementary Figure 6 for all genes with functional outside variants.
Supplementary Figure 8 Chromatin state of potential third variants.
Comparison of third SNPs that potentially explain the effect of outside variants on expression (see third arrow in Supplementary Fig. 7) to chromatin state. Proportion of these third SNPs that is significantly enriched for markers of regulatory function (H3K4me1, H3K27ac and DNase hypersensitivity) in B lymphoblasts (GM12878).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–3. (PDF 6186 kb)
Rights and permissions
About this article
Cite this article
Corradin, O., Cohen, A., Luppino, J. et al. Modeling disease risk through analysis of physical interactions between genetic variants within chromatin regulatory circuitry. Nat Genet 48, 1313–1320 (2016). https://doi.org/10.1038/ng.3674
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3674
This article is cited by
-
Three-dimensional genome: developmental technologies and applications in precision medicine
Journal of Human Genetics (2020)
-
A chromosomal connectome for psychiatric and metabolic risk variants in adult dopaminergic neurons
Genome Medicine (2020)
-
The Post-GWAS Era: How to Validate the Contribution of Gene Variants in Lupus
Current Rheumatology Reports (2019)
-
Bi-stream CNN Down Syndrome screening model based on genotyping array
BMC Medical Genomics (2018)
-
Identifying noncoding risk variants using disease-relevant gene regulatory networks
Nature Communications (2018)